Silica nanoparticle stability in biological media revisited
- PMID: 29317706
- PMCID: PMC5760698
- DOI: 10.1038/s41598-017-18502-8
Silica nanoparticle stability in biological media revisited
Abstract
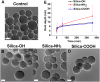
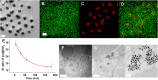
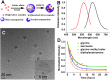
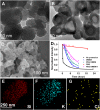
The stability of silica nanostructure in the core-silica shell nanomaterials is critical to understanding the activity of these nanomaterials since the exposure of core materials due to the poor stability of silica may cause misinterpretation of experiments, but unfortunately reports on the stability of silica have been inconsistent. Here, we show that luminescent silver nanodots (AgNDs) can be used to monitor the stability of silica nanostructures. Though relatively stable in water and phosphate buffered saline, silica nanoparticles are eroded by biological media, leading to the exposure of AgNDs from AgND@SiO2 nanoparticles and the quenching of nanodot luminescence. Our results reveal that a synergistic effect of organic compounds, particularly the amino groups, accelerates the erosion. Our work indicates that silica nanostructures are vulnerable to cellular medium and it may be possible to tune the release of drug molecules from silica-based drug delivery vehicles through controlled erosion.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Law WC, et al. Optically and magnetically doped organically modified silica nanoparticles as efficient magnetically guided biomarkers for two-photon imaging of live cancer cells. J. Phys. Chem. C. 2008;112:7972–7977. doi: 10.1021/jp712090y. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

