Shape dependent physical mutilation and lethal effects of silver nanoparticles on bacteria
- PMID: 29317760
- PMCID: PMC5760701
- DOI: 10.1038/s41598-017-18590-6
Shape dependent physical mutilation and lethal effects of silver nanoparticles on bacteria
Abstract
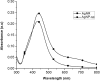
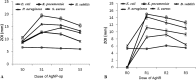
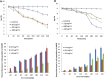
In this report, spherical silver nanoparticle (AgNP-sp) and rod-shaped silver nanoparticle (AgNR) were prepared by chemical reduction method and their antibacterial activity against various Gram-positive and Gram-negative bacteria had been evaluated for their efficiency. Minimal inhibitory concentration (MIC) tests were conducted to study the antibacterial properties, and substantiated with killing kinetics of silver nanoparticles (AgNPs). The study revealed that both AgNP-sp and AgNRs are good antibacterial candidates. Bacterial sensitivity to nanoparticles (NPs) was found to vary depending on microbial species. Disc diffusion studies revealed the greater effectiveness of AgNP-sp and AgNR against Klebsiella pneumoniae AWD5 at the doses of 249 and 392 µg. The dose dependent activities of prepared NPs were also observed on the batch studies of disc diffusion and MIC with various strains. The optical and morphological structures of NPs were analyzed by UV-visible, XRD, FE-SEM and TEM. Further, FESEM of bacterial culture treated with AgNPs confirmed antibacterial activity of NPs by showing rupture of bacterial cell wall. Also, the genome of test organism was found to have CusCFBA and CusRS operons. The killing kinetics confirmed that the death rate of K. pneumoniae was higher against AgNP-sp as compared to AgNR.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

