The impact of indacaterol/glycopyrronium fixed-dose combination versus tiotropium monotherapy on lung function and treatment preference: a randomized crossover study - the FAVOR study
- PMID: 29317812
- PMCID: PMC5744738
- DOI: 10.2147/COPD.S146189
The impact of indacaterol/glycopyrronium fixed-dose combination versus tiotropium monotherapy on lung function and treatment preference: a randomized crossover study - the FAVOR study
Abstract
Background: The objective of the FAVOR study was to evaluate the effect of indacaterol/glycopyrronium (IND/GLY) versus tiotropium on peak forced expiratory volume in 1 s (FEV1) and also to investigate patient satisfaction and treatment preference.
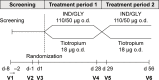
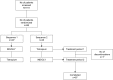
Methods: Patients with moderate-to-severe airflow limitation (FEV1/forced vital capacity ratio of <0.70), those with a COPD assessment test score of ≥10, and those who were maintained on tiotropium HandiHaler® therapy prior to enrollment were recruited for the study, and randomized (1:1) to receive either 4 weeks open-label IND/GLY (110/50 μg) once daily followed by 4 weeks of tiotropium (18 μg) once daily or vice versa. The primary endpoint was FEV1 1 h post-inhalation after 4 weeks of treatment. Other endpoints included patient's and physician's preference for treatment, patient's satisfaction evaluated using a study-specific questionnaire and the abbreviated Treatment Satisfaction Questionnaire for Medication, and safety and tolerability.
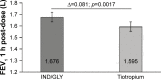
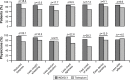
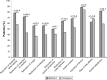
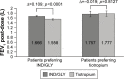
Results: Eighty-seven out of 88 randomized patients completed the study and showed significantly higher FEV1 1 h post-inhalation after 4 weeks of treatment with IND/GLY versus tiotropium (treatment difference =0.081 L; p=0.0017). IND/GLY was preferred over tiotropium among the patients (69.4% versus 30.6%, p=0.0004) and the physicians (81.6% versus 18.4%, p<0.0001). A higher proportion of the patients stated they were very satisfied or satisfied with IND/GLY versus tiotropium with regard to dyspnea reduction (79.3% versus 58.0%, respectively) and reduction of dyspnea on exertion (72.4% versus 43.2%, respectively). Patients treated with IND/GLY showed significant improvement in Treatment Satisfaction Questionnaire for Medication domain scores versus tiotropium. IND/GLY demonstrated a good safety and tolerability profile.
Conclusion: This study indicated that, beyond FEV1, important patient-reported outcomes improved with the open-label dual bronchodilator IND/GLY when compared with tiotropium. This study suggests that individual patients felt the lung function benefits with IND/GLY compared with tiotropium, which, in turn, may also have contributed to the preference for IND/GLY.
Keywords: COPD; FEV1; dual bronchodilators; indacaterol/glycopyrronium; treatment choice; treatment preference.
Conflict of interest statement
Disclosure Dr Peter Kardos reports personal fees and study honoraria for his practice from Novartis during the conduct of the study; personal fees and other from AstraZeneca and Boehringer Ingelheim; and personal fees from Chiesi, GSK, Menarini, and Teva, outside the submitted work. Dr Ina Hagedorn-Peinz is an employee of Novartis Pharma GmbH and was in a medical role during the study conduct and in a commercial role during the manuscript development. The authors report no other conflicts of interest in this work.
Figures

References
-
- Tashkin DP. Long-acting anticholinergic use in chronic obstructive pulmonary disease: efficacy and safety. Curr Opin Pulm Med. 2010;16(2):97–105. - PubMed
-
- World Health Organization The top 10 causes of death. [webpage on the Internet] [Accessed August 17, 2017]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/
-
- GOLD Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2016. [Accessed August 17, 2017]. Available from: www.goldcopd.org.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

