Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of Gi-protein inhibitor
- PMID: 29317816
- PMCID: PMC5743190
- DOI: 10.2147/IJN.S150918
Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of Gi-protein inhibitor
Abstract
Background: Lipopolysaccharide (LPS) is widely recognized as a potent activator of monocytes/macrophages, and its effects include an altered production of key mediators, such as inflammatory cytokines and chemokines. The involvement of Gi protein in mediating LPS effects has been demonstrated in murine macrophages and various cell types of human origin.
Purpose: The aim of the present work was to evaluate the potential of a Gi-protein inhibitor encapsulated in liposomes in reducing the inflammatory effects induced by LPS in monocytes/macrophages.
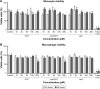
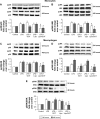
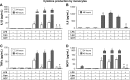
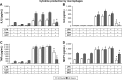
Materials and methods: Guanosine 5'-O-(2-thiodiphosphate) (GOT), a guanosine diphosphate analog that completely inhibits G-protein activation by guanosine triphosphate and its analogs, was encapsulated into liposomes and tested for anti-inflammatory effects in LPS-activated THP1 monocytes or THP1-derived macrophages. The viability of monocytes/macrophages after incubation with different concentrations of free GOT or liposome-encapsulated GOT was assessed by MTT assay. MAPK activation and production of IL1β, TNFα, IL6, and MCP1 were assessed in LPS-activated monocytes/macrophages in the presence or absence of free or encapsulated GOT. In addition, the effect of free or liposome-encapsulated GOT on LPS-stimulated monocyte adhesion to activated endothelium and on monocyte chemotaxis was evaluated.
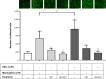
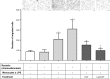
Results: We report here that GOT-loaded liposomes inhibited activation of MAPK and blocked the production of the cytokines IL1β, TNFα, IL6, and MCP1 induced by LPS in monocytes and macrophages. Moreover, GOT encapsulated in liposomes reduced monocyte adhesion and chemotaxis. All demonstrated events were in contrast with free GOT, which showed reduced or no effect on monocyte/macrophage activation with LPS.
Conclusion: This study demonstrates the potential of liposomal GOT in blocking LPS proinflammatory effects in monocytes/macrophages.
Keywords: MAPK activation; chemotaxis; cytokine; guanosine 5′-O-(2-thiodiphosphate) (GOT); monocyte adhesion.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Calin M, Manduteanu I. Emerging nanocarriers-based approaches to diagnose and reduce vascular inflammation in atherosclerosis. Curr Med Chem. 2017;24:550–567. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

