Tacrolimus nanoparticles based on chitosan combined with nicotinamide: enhancing percutaneous delivery and treatment efficacy for atopic dermatitis and reducing dose
- PMID: 29317821
- PMCID: PMC5743175
- DOI: 10.2147/IJN.S150319
Tacrolimus nanoparticles based on chitosan combined with nicotinamide: enhancing percutaneous delivery and treatment efficacy for atopic dermatitis and reducing dose
Abstract
Background: Topical application of tacrolimus (FK506) was effective in the treatment of atopic dermatitis (AD); however, adverse effects frequently occurred with the increase of FK506 dose during long-term treatment.
Objective: The objective of this project was to develop a hybrid skin targeting system encapsulating FK506 based on nicotinamide (NIC) and chitosan nanoparticles (CS-NPs), ie, FK506-NIC-CS-NPs, which took advantages of both of NIC and CS-NPs to obtain the synergetic effects of percutaneous delivery and treatment efficacy enhancement along with dose reduction.
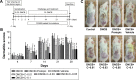
Methods: The formulation of FK506-NIC-CS-NPs was optimized and characterized. In vitro and in vivo skin permeation studies were performed. AD-like skin lesions were constructed with BALB/c mice by 1-chloro-2, 4-dinitrobenzene (DNCB)-induced, and FK506-NIC-CS-NPs containing different dose of FK506 were topically administered to treat AD-like skin lesions in comparison with Protopic.
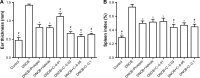
Results: NIC was found to significantly increase the FK506 EE to 92.2% by CS-NPs. In comparison with commercial FK506 ointment (Protopic), in vitro and in vivo skin permeation studies demonstrated that NIC-CS-NPs system significantly enhanced FK506 permeation through and into the skin, and deposited more FK506 into the skin. The treatment efficacy on clinical symptoms, histological analysis, and molecular biology of the AD-mice demonstrated that NIC-CS-NPs with ~1/3 dose of FK506 of Protopic was superior to that of Protopic, and NIC-CS-NPs vehicle exhibited the adjuvant therapy and moderate anti-AD effects.
Conclusion: The system of NIC-CS-NPs enhances the permeability of FK506, plays an adjuvant role in anti-AD, reduces the dose of FK506 in treating AD, and is therefore a promising nanoscale system of FK506 for the effective treatment of AD.
Keywords: atopic dermatitis; chitosan; nanoparticles; nicotinamide; percutaneous delivery; reducing dose; tacrolimus.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures





References
-
- Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(6 Suppl):S118–S127. - PubMed
-
- Soter NA. Morphology of atopic eczema. Allergy. 1989;44(Suppl 9):16–19. - PubMed
-
- Abboud D, Hanson J. Chemokine neutralization as an innovative therapeutic strategy for atopic dermatitis. Drug Discov Today. 2017;22(4):702–711. - PubMed
-
- Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361(9352):151–160. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

