Novel functionalized nanoparticles for tumor-targeting co-delivery of doxorubicin and siRNA to enhance cancer therapy
- PMID: 29317822
- PMCID: PMC5743186
- DOI: 10.2147/IJN.S148960
Novel functionalized nanoparticles for tumor-targeting co-delivery of doxorubicin and siRNA to enhance cancer therapy
Abstract
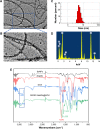
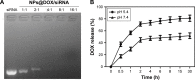
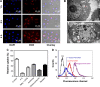
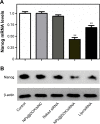
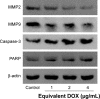
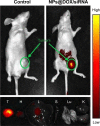
Human homeobox protein (Nanog) is highly expressed in most cancer cells and has gradually emerged as an excellent target in cancer therapy, owing to its regulation of cancer cell proliferation, metastasis and apoptosis. In this study, we prepared tumor-targeting functionalized selenium nanoparticles (RGDfC-SeNPs) to load chemotherapeutic doxorubicin (DOX) and Nanog siRNA. Herein, RGDfC peptide was used as a tumor-targeting moiety which could specifically bind to αvβ3 integrins overexpressed on various cancer cells. The sizes of RGDfC-SeNPs@DOX nanoparticles (~12 nm) were confirmed by both dynamic light scattering and transmission electron microscopy. The chemical structure of RGDfC-SeNPs@DOX was characterized via Fourier-transform infrared spectroscopy. The RGDfC-SeNPs@DOX was compacted with siRNA (anti-Nanog) by electrostatic interaction to fabricate the RGDfC-SeNPs@DOX/siRNA complex. The RGDfC-SeNPs@DOX/siRNA complex nanoparticles could efficiently enter into HepG2 cells via clathrin-associated endocytosis, and showed high gene transfection efficiency that resulted in enhanced gene silencing. The in vivo biodistribution experiment indicated that RGDfC-SeNPs@DOX/siRNA nanoparticles were capable of specifically accumulating in the tumor site. Furthermore, treatment with RGDfC-SeNPs@DOX/siRNA resulted in a more significant anticancer activity than the free DOX, RGDfC-SeNPs@DOX or RGDfC-SeNPs/siRNA in vitro and in vivo. In summary, this study shows a novel type of DOX and siRNA co-delivery system, thereby providing an alternative route for cancer treatment.
Keywords: Nanog siRNA; doxorubicin; drug delivery; nanoparticles; tumor targeting.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures





References
-
- Ramasamy T, Ruttala HB, Gupta B, et al. Smart chemistry-based nanosized drug delivery systems for systemic applications: a comprehensive review. J Control Release. 2017;258(8):226–253. - PubMed
-
- Lin X, Yang S, Lai K, Yang H, Webster TJ, Yang L. Orthopedic implant biomaterials with both osteogenic and anti-infection capacities and associated in vivo evaluation methods. Nanomedicine. 2017;13(1):123–142. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

