Adverse Renal Effects of Novel Molecular Oncologic Targeted Therapies: A Narrative Review
- PMID: 29318210
- PMCID: PMC5720524
- DOI: 10.1016/j.ekir.2016.09.055
Adverse Renal Effects of Novel Molecular Oncologic Targeted Therapies: A Narrative Review
Abstract
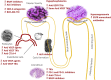
Novel targeted anti-cancer therapies have resulted in improvement in patient survival compared to standard chemotherapy. Renal toxicities of targeted agents are increasingly being recognized. The incidence, severity, and pattern of renal toxicities may vary according to the respective target of the drug. Here we review the adverse renal effects associated with a selection of currently approved targeted cancer therapies, directed to EGFR, HER2, BRAF, MEK, ALK, PD1/PDL1, CTLA-4, and novel agents targeted to VEGF/R and TKIs. In summary, electrolyte disorders, renal impairment and hypertension are the most commonly reported events. Of the novel targeted agents, ipilumumab and cetuximab have the most nephrotoxic events reported. The early diagnosis and prompt recognition of these renal adverse events are essential for the general nephrologist taking care of these patients.
Keywords: AKI; chemotherapy; hypokalemia; hyponatremia; nephrotoxicity; onconephrology; renal failure; targeted therapy.
Figures

References
-
- National Cancer Institute. Targeted therapy. Available at: http://www.cancer.gov/about-cancer/treatment/types/targeted-therapies. Accessed January 1, 2016.
-
- Jhaveri K.D., Sakhiya V.B., Wanchoo R. Renal effects of novel anti cancer targeted therapies: a review of the FDA Adverse Event Reporting System. Kidney Int. 2016;90:705–707. - PubMed
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. - PubMed
-
- Gurevich F., Perazella M.A. Renal effects of anti-angiogenesis therapy: update for the internist. Am J Med. 2009;122:322–328. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

