Razor-printed sticker microdevices for cell-based applications
- PMID: 29318250
- PMCID: PMC5821501
- DOI: 10.1039/c7lc00724h
Razor-printed sticker microdevices for cell-based applications
Abstract
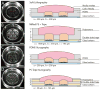
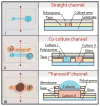
Tape-based razor-printing is a flexible and affordable ultra-rapid prototyping approach for microscale device fabrication. However, integration of this prototyping approach into cell-based assay development has been limited to proof of principle demonstrations. This is in large part due to lack of an established or well-characterized option for biocompatible adhesive tape. Without such an option, integration of these areas will remain unexplored. Therefore, to address this critical hurdle, we characterized microscale devices made using a potentially biocompatible double-sided adhesive, ARCare 90106. We validated tape-based device performance against 96-well plates and PDMS microdevices with respect to cell viability, hydrophobic small molecule sequestration, the potential for leaching compounds, use in fluorescence microscopy, and outgassing (bubble formation). Results supported the tape as a promising tool for future cell-based assay development. Therefore, we subsequently demonstrated specific strengths enabled by the ultra-rapid (<1 h per prototype) and affordable (∼$1200 cutting plotter, <$0.05 per prototype) approach. Specifically, data demonstrate the ability to integrate disparate materials for advanced sticker-device functionality such as bonding of polystyrene devices to glass substrates for microscopy applications, inclusion of membranes, and incorporation of different electrospun biomaterials into a single device. Likewise, the approach allowed rapid adoption by uninitiated users. Overall, this study provides a necessary and unique contribution to the largely separate fields of tape-based razor-printing and cell-based microscale assay development by addressing a critical barrier to widespread integration and adoption while also demonstrating the potential for new and future applications.
Figures





References
-
- Cosson S, Aeberli LG, Brandenberg N, Lutolf MP. Ultra-rapid prototyping of flexible, multi-layered microfluidic devices via razor writing. Lab Chip. 2014;15:72–76. - PubMed
-
- de Santana PP, de Oliveira IMF, Piccin E. Evaluation of using xurography as a new technique for the fabrication of disposable gold electrodes with highly reproducible areas. Electrochem commun. 2012;16:96–99.
-
- Greer J, Sundberg SO, Wittwer CT, Gale BK. Comparison of glass etching to xurography prototyping of microfluidic channels for DNA melting analysis. J Micromech Microeng. 2007;17:2407–2413.
-
- Renaud L, Selloum D, Tingry S. Xurography for 2D and multi-level glucose/O2 microfluidic biofuel cell. Microfluid Nanofluidics. 2015;18:1407–1416.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

