Comparison of oral montelukast with oral ozagrel in acute asthma: A randomized, double-blind, placebo-controlled study
- PMID: 29319028
- PMCID: PMC5760861
- DOI: 10.4103/lungindia.lungindia_226_17
Comparison of oral montelukast with oral ozagrel in acute asthma: A randomized, double-blind, placebo-controlled study
Abstract
Background: The need for more effective management of acute asthma has led to research on drugs which are otherwise approved for use in chronic asthma.
Objective: To study and compare the effects of oral montelukast with oral ozagrel in acute asthma.
Materials and methods: One hundred and twenty patients with acute asthma were recruited for the study. Out of 120 study patients, forty each were randomized into placebo, montelukast, and ozagrel groups. After the first dose of the drug or placebo was administered, peak expiratory flow rate (PEFR), number of rescue medications and also vital signs were noted at 6 h, 12 h, 24 h, 48 h, and at discharge. In addition, same recordings were done on the morning (8 a.m. - 10 a.m.) following admission. The difference in mean PEFR of each group at above-mentioned time points was the primary endpoint whereas need for rescue medications the secondary end-point.
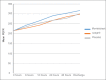
Results: The respective mean PEFR recordings of the placebo, montelukast, and ozagrel groups at various time points were as follows: at 6 h (235.19 ± 3.18, 242.86 ± 3.26, 228.18 ± 3.25); at 12 h (254.37 ± 5.23, 265.62 ± 5.38, 242.99 ± 5.36); at 24 h (267.46 ± 7.41, 291.39 ± 7.61, 268.14 ± 7.58); and at 48 h (277.99 ± 7.35, 303.22 ± 7.56, 285.27 ± 7.53); and discharge (301.94 ± 7.07, 317.32 ± 7.27, 298.99 ± 7.23). The mean PEFR between the treatment groups were not statistically significant (P = 0.102). The mean PEFR in the three groups at 8-10 a.m. following admission was 257.60 ± 5.52, 264.23 ± 5.98, and 249.94 ± 5.96; P = 0.266. Total number of rescue doses needed were 7, 4, and 13, respectively (P = 0.67).
Conclusion: Montelukast or ozagrel when added to the standard treatment of acute asthma does not result in any additional benefit.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Ramsay CF, Pearson D, Mildenhall S, Wilson AM. Oral montelukast in acute asthma exacerbations: A randomised, double-blind, placebo-controlled trial. Thorax. 2011;66:7–11. - PubMed
-
- Salvi SS, Krishna MT, Sampson AP, Holgate ST. The anti-inflammatory effects of leukotriene-modifying drugs and their use in asthma. Chest. 2001;119:1533–46. - PubMed
-
- Myou S, Fujimura M, Leff AR. Additive effect of cysteinyl leukotriene or thromboxane modifiers to inhaled corticosteroids in asthmatic patients. Allergol Int. 2004;53:211–7.
-
- British Thoracic Society. Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax. 2014;69(Suppl 1):1–192. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources