N-Acetyl-2-Aminofluorene (AAF) Processing in Adult Rat Hepatocytes in Primary Culture Occurs by High-Affinity Low-Velocity and Low-Affinity High-Velocity AAF Metabolite-Forming Systems
- PMID: 29319795
- PMCID: PMC5917773
- DOI: 10.1093/toxsci/kfy006
N-Acetyl-2-Aminofluorene (AAF) Processing in Adult Rat Hepatocytes in Primary Culture Occurs by High-Affinity Low-Velocity and Low-Affinity High-Velocity AAF Metabolite-Forming Systems
Erratum in
-
Erratum to "N-Acetyl-2-Aminofluorene (AAF) Processing in Adult Rat Hepatocytes in Primary Culture Occurs by High-Affinity Low-Velocity and Low-Affinity High-Velocity AAF Metabolite-Forming Systems" and "High-Affinity Low-Capacity and Low-Affinity High-Capacity N-Acetyl-2-Aminofluorene (AAF) Macromolecular Binding Sites Are Revealed During the Growth Cycle of Adult Rat Hepatocytes in Primary Culture".Toxicol Sci. 2018 May 25;163(2):667. doi: 10.1093/toxsci/kfy118. Online ahead of print. Toxicol Sci. 2018. PMID: 29846706 Free PMC article. No abstract available.
Abstract
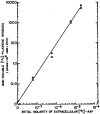
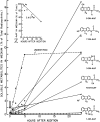
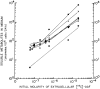
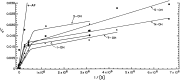
N-acetyl-2-aminofluorene (AAF) is a procarcinogen used widely in physiological investigations of chemical hepatocarcinogenesis. Its metabolic pathways have been described extensively, yet little is known about its biochemical processing, growth cycle expression, and pharmacological properties inside living hepatocytes-the principal cellular targets of this hepatocarcinogen. In this report, primary monolayer adult rat hepatocyte cultures and high specific-activity [ring G-3 H]-N-acetyl-2-aminofluorene were used to extend previous observations of metabolic activation of AAF by highly differentiated, proliferation-competent hepatocytes in long-term cultures. AAF metabolism proceeded by zero-order kinetics. Hepatocytes processed significant amounts of procarcinogen (≈12 μg AAF/106 cells/day). Five ring-hydroxylated and one deacetylated species of AAF were secreted into the culture media. Extracellular metabolite levels varied during the growth cycle (days 0-13), but their rank quantitative order was time invariant: 5-OH-AAF > 7-OH-AAF > 3-OH-AAF > N-OH-AAF > aminofluorene (AF) > 1-OH-AAF. Lineweaver-Burk analyses revealed two principal classes of metabolism: System I (high-affinity and low-velocity), Km[APPARENT] = 1.64 × 10-7 M and VMAX[APPARENT] = 0.1 nmol/106 cells/day and System II (low-affinity and high-velocity), Km[APPARENT] = 3.25 × 10-5 M and VMAX[APPARENT] = 1000 nmol/106 cells/day. A third system of metabolism of AAF to AF, with Km[APPARENT] and VMAX[APPARENT] constants of 9.6 × 10-5 M and 4.7 nmol/106 cells/day, was also observed. Evidence provided in this report and its companion paper suggests selective roles and intracellular locations for System I- and System II-mediated AAF metabolite formation during hepatocarcinogenesis, although some of the molecules and mechanisms responsible for multi-system processing remain to be fully defined.
Figures


References
-
- American Cancer Society. (2018). Liver Cancer. http://www.cancer. org/acs/groups/cid/documents/webcontent/003114-pdf.pdf
-
- Annalora A. J., Marcus C. B., Iversen P. L. (2017). Alternative splicing in the cytochrome P450 superfamily expands protein diversity to augment gene function and redirect human drug metabolism. Drug Metab. Dispos. 45, 375–389.http://dx.doi.org/10.1124/dmd.116.073254 - DOI - PubMed
-
- Aström A., Birberg W., Pilotti A., DePierre J. W. (1986). Induction of different isozymes of cytochrome P-450 and of microsomal epoxide hydrolase in rat liver by 2-acetylaminofluorene and structurally related compounds. Eur. J. Biochem. 154, 125–134. - PubMed
-
- Bartsch H., Dworkin M., Miller J. A., Miller E. C. (1972). Electrophilic N-acetoxyaminoarenes derived from carcinogenic N-hydroxy-N-acetylaminoarenes by enzymatic deacetylation and transacetylation in liver. Biochim. Biophys. Acta 286, 272–298. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

