Differentially Expressed mRNA Targets of Differentially Expressed miRNAs Predict Changes in the TP53 Axis and Carcinogenesis-Related Pathways in Human Keratinocytes Chronically Exposed to Arsenic
- PMID: 29319823
- PMCID: PMC5889014
- DOI: 10.1093/toxsci/kfx292
Differentially Expressed mRNA Targets of Differentially Expressed miRNAs Predict Changes in the TP53 Axis and Carcinogenesis-Related Pathways in Human Keratinocytes Chronically Exposed to Arsenic
Abstract
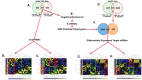
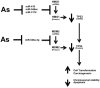
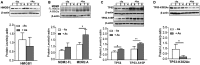
Arsenic is a widely distributed toxic natural element. Chronic arsenic ingestion causes several cancers, especially skin cancer. Arsenic-induced cancer mechanisms are not well defined, but several studies indicate that mutation is not the driving force and that microRNA expression changes play a role. Chronic low arsenite exposure malignantly transforms immortalized human keratinocytes (HaCaT), serving as a model for arsenic-induced skin carcinogenesis. Early changes in miRNA expression in HaCaT cells chronically exposed to arsenite will reveal early steps in transformation. HaCaT cells were maintained with 0/100 nM NaAsO2 for 3 and 7 weeks. Total RNA was purified. miRNA and mRNA expression was assayed using Affymetrix microarrays. Targets of differentially expressed miRNAs were collected from TargetScan 6.2, intersected with differentially expressed mRNAs using Partek Genomic Suite software, and mapped to their pathways using MetaCore software. MDM2, HMGB1 and TP53 mRNA, and protein levels were assayed by RT-qPCR and Western blot. Numerous miRNAs and mRNAs involved in carcinogenesis pathways in other systems were differentially expressed at 3 and 7 weeks. A TP53 regulatory network including MDM2 and HMGB1 was predicted by the miRNA and mRNA networks. Total TP53 and TP53-S15-phosphorylation were induced. However, TP53-K382-hypoacetylation suggested that the induced TP53 is inactive in arsenic exposed cells. Our data provide strong evidence that early changes in miRNAs and target mRNAs may contribute to arsenic-induced carcinogenesis.
Figures
References
-
- Agency for Toxic Substances and Disease Registry. (2014). Priority List of Hazardous Substances. Available at: http://www.atsdr.cdc.gov/spl/index.html. Accessed June 2016.
-
- American Cancer Society. (2014). Arsenic. Available at: http://www.cancer.org/cancer/cancercauses/othercarcinogens/intheworkplac.... Accessed June 2016.
-
- Anetor J. I., Wanibuchi H., Fukushima S. (2007). Arsenic exposure and its health effects and risk of cancer in developing countries: micronutrients as host defence. Asian Pac J Cancer Prev 8, 13–23. - PubMed
-
- Bartel F., Meye A., Wurl P., Kappler M., Bache M., Lautenschlager C., Grunbaum U., Schmidt H., Taubert H. (2001). Amplification of the MDM2 gene, but not expression of splice variants of MDM2 MRNA, is associated with prognosis in soft tissue sarcoma. Int. J. Cancer 95, 168–175. - PubMed
-
- Bartel F., Taubert H., Harris L. C. (2002). Alternative and aberrant splicing of MDM2 mRNA in human cancer. Cancer Cell 2, 9–15.http://dx.doi.org/10.1016/S1535-6108(02)00091-0 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

