An extracellular network of Arabidopsis leucine-rich repeat receptor kinases
- PMID: 29320478
- PMCID: PMC6485605
- DOI: 10.1038/nature25184
An extracellular network of Arabidopsis leucine-rich repeat receptor kinases
Erratum in
-
Publisher Correction: An extracellular network of Arabidopsis leucine-rich repeat receptor kinases.Nature. 2018 Sep;561(7722):E8. doi: 10.1038/s41586-018-0268-y. Nature. 2018. PMID: 29973716
Abstract
The cells of multicellular organisms receive extracellular signals using surface receptors. The extracellular domains (ECDs) of cell surface receptors function as interaction platforms, and as regulatory modules of receptor activation. Understanding how interactions between ECDs produce signal-competent receptor complexes is challenging because of their low biochemical tractability. In plants, the discovery of ECD interactions is complicated by the massive expansion of receptor families, which creates tremendous potential for changeover in receptor interactions. The largest of these families in Arabidopsis thaliana consists of 225 evolutionarily related leucine-rich repeat receptor kinases (LRR-RKs), which function in the sensing of microorganisms, cell expansion, stomata development and stem-cell maintenance. Although the principles that govern LRR-RK signalling activation are emerging, the systems-level organization of this family of proteins is unknown. Here, to address this, we investigated 40,000 potential ECD interactions using a sensitized high-throughput interaction assay, and produced an LRR-based cell surface interaction network (CSILRR) that consists of 567 interactions. To demonstrate the power of CSILRR for detecting biologically relevant interactions, we predicted and validated the functions of uncharacterized LRR-RKs in plant growth and immunity. In addition, we show that CSILRR operates as a unified regulatory network in which the LRR-RKs most crucial for its overall structure are required to prevent the aberrant signalling of receptors that are several network-steps away. Thus, plants have evolved LRR-RK networks to process extracellular signals into carefully balanced responses.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


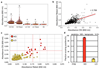




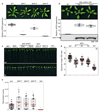


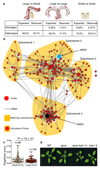
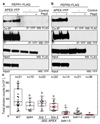

Comment in
-
Antenna network.Nat Plants. 2018 Feb;4(2):61. doi: 10.1038/s41477-018-0111-3. Nat Plants. 2018. PMID: 29379146 No abstract available.
-
The APEX Approaches: A Unified LRR-RK Network Revealed.Trends Plant Sci. 2018 May;23(5):372-374. doi: 10.1016/j.tplants.2018.03.008. Epub 2018 Mar 27. Trends Plant Sci. 2018. PMID: 29602571 Free PMC article.
Similar articles
-
Mechanistic basis for the activation of plant membrane receptor kinases by SERK-family coreceptors.Proc Natl Acad Sci U S A. 2018 Mar 27;115(13):3488-3493. doi: 10.1073/pnas.1714972115. Epub 2018 Mar 12. Proc Natl Acad Sci U S A. 2018. PMID: 29531026 Free PMC article.
-
The EDS1-PAD4-ADR1 node mediates Arabidopsis pattern-triggered immunity.Nature. 2021 Oct;598(7881):495-499. doi: 10.1038/s41586-021-03829-0. Epub 2021 Sep 8. Nature. 2021. PMID: 34497423
-
Map of physical interactions between extracellular domains of Arabidopsis leucine-rich repeat receptor kinases.Sci Data. 2019 Feb 26;6:190025. doi: 10.1038/sdata.2019.25. Sci Data. 2019. PMID: 30806640 Free PMC article.
-
Plant Leucine-Rich Repeat Receptor Kinase (LRR-RK): Structure, Ligand Perception, and Activation Mechanism.Molecules. 2019 Aug 25;24(17):3081. doi: 10.3390/molecules24173081. Molecules. 2019. PMID: 31450667 Free PMC article. Review.
-
Leucine-rich repeat receptor kinases in plants: structure, function, and signal transduction pathways.Int Rev Cytol. 2004;234:1-46. doi: 10.1016/S0074-7696(04)34001-5. Int Rev Cytol. 2004. PMID: 15066372 Review.
Cited by
-
An Interactome Assay for Detecting Interactions between Extracellular Domains of Receptor Kinases.Methods Mol Biol. 2023;2690:193-204. doi: 10.1007/978-1-0716-3327-4_18. Methods Mol Biol. 2023. PMID: 37450149
-
Laying it on thick: a study in secondary growth.J Exp Bot. 2022 Jan 27;73(3):665-679. doi: 10.1093/jxb/erab455. J Exp Bot. 2022. PMID: 34655214 Free PMC article. Review.
-
The root-knot nematode Meloidogyne incognita produces a functional mimic of the Arabidopsis INFLORESCENCE DEFICIENT IN ABSCISSION signaling peptide.J Exp Bot. 2018 May 25;69(12):3009-3021. doi: 10.1093/jxb/ery135. J Exp Bot. 2018. PMID: 29648636 Free PMC article.
-
Regulation of immune receptor kinase plasma membrane nanoscale organization by a plant peptide hormone and its receptors.Elife. 2022 Jan 6;11:e74162. doi: 10.7554/eLife.74162. Elife. 2022. PMID: 34989334 Free PMC article.
-
The immune NIK1/RPL10/LIMYB signaling module regulates photosynthesis and translation under biotic and abiotic stresses.Nat Commun. 2025 May 13;16(1):4433. doi: 10.1038/s41467-025-59571-y. Nat Commun. 2025. PMID: 40360515 Free PMC article.
References
-
- Jaillais Y, Belkhadir Y, Balsemao-Pires E, Dangl JL, Chory J. Extracellular leucine-rich repeats as a platform for receptor/coreceptor complex formation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8503–8507. doi: 10.1073/pnas.1103556108. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

