M1-like macrophages change tumor blood vessels and microenvironment in murine melanoma
- PMID: 29320562
- PMCID: PMC5761928
- DOI: 10.1371/journal.pone.0191012
M1-like macrophages change tumor blood vessels and microenvironment in murine melanoma
Abstract
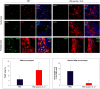
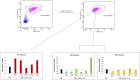
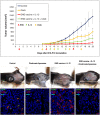
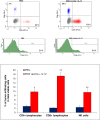
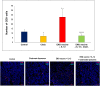
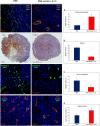
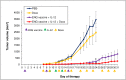
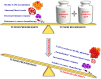
Tumor-associated macrophages (TAMs) play a significant role in at least two key processes underlying neoplastic progression: angiogenesis and immune surveillance. TAMs phenotypic changes play important role in tumor vessel abnormalization/ normalization. M2-like TAMs stimulate immunosuppression and formation of defective tumor blood vessels leading to tumor progression. In contrast M1-like TAMs trigger immune response and normalize irregular tumor vascular network which should sensitize cancer cells to chemo- and radiotherapy and lead to tumor growth regression. Here, we demonstrated that combination of endoglin-based DNA vaccine with interleukin 12 repolarizes TAMs from tumor growth-promoting M2-like phenotype to tumor growth-inhibiting M1-like phenotype. Combined therapy enhances tumor infiltration by CD4+, CD8+ lymphocytes and NK cells. Depletion of TAMs as well as CD8+ lymphocytes and NK cells, but not CD4+ lymphocytes, reduces the effect of combined therapy. Furthermore, combined therapy improves tumor vessel maturation, perfusion and reduces hypoxia. It caused that suboptimal doses of doxorubicin reduced the growth of tumors in mice treated with combined therapy. To summarize, combination of antiangiogenic drug and immunostimulatory agent repolarizes TAMs phenotype from M2-like (pro-tumor) into M1-like (anti-tumor) which affects the structure of tumor blood vessels, improves the effect of chemotherapy and leads to tumor growth regression.
Conflict of interest statement
Figures



Similar articles
-
The prednisolone phosphate‑induced suppression of the angiogenic function of tumor‑associated macrophages enhances the antitumor effects of doxorubicin on B16.F10 murine melanoma cells in vitro.Oncol Rep. 2019 Dec;42(6):2694-2705. doi: 10.3892/or.2019.7346. Epub 2019 Oct 1. Oncol Rep. 2019. PMID: 31578578
-
Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes.Theranostics. 2021 Jan 1;11(6):2892-2916. doi: 10.7150/thno.50928. eCollection 2021. Theranostics. 2021. PMID: 33456579 Free PMC article.
-
Antitumor effects of recombinant antivascular protein ABRaA-VEGF121 combined with IL-12 gene therapy.Arch Immunol Ther Exp (Warsz). 2014 Apr;62(2):161-8. doi: 10.1007/s00005-013-0259-5. Epub 2013 Nov 13. Arch Immunol Ther Exp (Warsz). 2014. PMID: 24220932 Free PMC article.
-
Tumor promoting role of anti-tumor macrophages in tumor microenvironment.Cell Immunol. 2017 Jun;316:1-10. doi: 10.1016/j.cellimm.2017.04.005. Epub 2017 Apr 13. Cell Immunol. 2017. PMID: 28433198 Review.
-
The role of tumor-associated macrophages in gastric cancer development and their potential as a therapeutic target.Cancer Treat Rev. 2020 Jun;86:102015. doi: 10.1016/j.ctrv.2020.102015. Epub 2020 Mar 23. Cancer Treat Rev. 2020. PMID: 32248000 Review.
Cited by
-
Macrophages in melanoma: A double‑edged sword and targeted therapy strategies (Review).Exp Ther Med. 2022 Aug 26;24(4):640. doi: 10.3892/etm.2022.11577. eCollection 2022 Oct. Exp Ther Med. 2022. PMID: 36160877 Free PMC article. Review.
-
Interaction of tumor‑associated macrophages with stromal and immune components in solid tumors: Research progress (Review).Int J Oncol. 2023 Feb;62(2):32. doi: 10.3892/ijo.2023.5480. Epub 2023 Jan 20. Int J Oncol. 2023. PMID: 36660926 Free PMC article. Review.
-
CSC Radioresistance: A Therapeutic Challenge to Improve Radiotherapy Effectiveness in Cancer.Cells. 2020 Jul 9;9(7):1651. doi: 10.3390/cells9071651. Cells. 2020. PMID: 32660072 Free PMC article. Review.
-
Histidine-rich glycoprotein-induced vascular normalization improves EPR-mediated drug targeting to and into tumors.J Control Release. 2018 Jul 28;282:25-34. doi: 10.1016/j.jconrel.2018.05.002. Epub 2018 May 4. J Control Release. 2018. PMID: 29730154 Free PMC article.
-
Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers.Nat Commun. 2019 Sep 3;10(1):3974. doi: 10.1038/s41467-019-11911-5. Nat Commun. 2019. PMID: 31481662 Free PMC article.
References
-
- Szala S, Mitrus I, Sochanik A. Can inhibition of angiogenesis and stimulation of immune response be combined into a more effective antitumor therapy? Cancer Immunol Immunother. 2010;59: 1449–1455. doi: 10.1007/s00262-010-0873-6 - DOI - PMC - PubMed
-
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21: 309–322. doi: 10.1016/j.ccr.2012.02.022 - DOI - PubMed
-
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19: 1423–1437. doi: 10.1038/nm.3394 - DOI - PMC - PubMed
-
- Hui L, Chen Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015;368: 7–13. doi: 10.1016/j.canlet.2015.07.039 - DOI - PubMed
-
- Szala S, Jarosz M, Cichoń T, Smolarczyk R, Sochanik A. Polarization of tumor milieu: therapeutic implications In: Cancer Immunology: Translational Medicine from Bench to Bedside. Part II: Cancer Immunotherapy. Springer-Verlag; Berlin Heidelberg: 2015. pp. 401–408. https://doi.org/10.1007/978-3-662-44946-2_22. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

