AZP-531, an unacylated ghrelin analog, improves food-related behavior in patients with Prader-Willi syndrome: A randomized placebo-controlled trial
- PMID: 29320575
- PMCID: PMC5761957
- DOI: 10.1371/journal.pone.0190849
AZP-531, an unacylated ghrelin analog, improves food-related behavior in patients with Prader-Willi syndrome: A randomized placebo-controlled trial
Abstract
Context and objective: Prader-Willi syndrome (PWS) is characterized by early-onset hyperphagia and increased circulating levels of the orexigenic Acylated Ghrelin (AG) hormone with a relative deficit of Unacylated Ghrelin (UAG). AZP-531, a first-in-class UAG analog, was shown to inhibit the orexigenic effect of AG in animals, to improve glycemic control and decrease body weight in humans. We aimed to investigate the safety and efficacy of AZP-531 in patients with PWS for whom no approved treatment for hyperphagia is currently available.
Methods and design: Multi-center, randomized, double-blind, placebo-controlled trial. Forty-seven patients with genetically confirmed PWS and evidence of hyperphagia received daily subcutaneous injections of AZP-531 (3 and 4 mg for 50-70 kg and >70 kg body weight, respectively) or matching placebo for 14 days. Assessments included adverse events, vital signs, safety laboratory tests, the Hyperphagia Questionnaire (HQ), patient-reported appetite, body composition and glycemic measures.
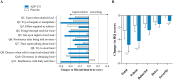
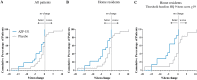
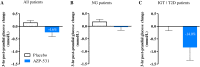
Results: AZP-531 was well tolerated. There was a significant improvement with AZP-531 versus placebo in the mean total score, the 9-item score and the severity domain score of the HQ (p < .05). The highest reduction in the total and 9-item scores was observed in AZP-531 subjects with the highest hyperphagia score at baseline. Findings were supported by a reduction in appetite scores observed with AZP-531 only. Body weight did not change in both groups while a significant reduction in waist circumference and fat mass was observed only with AZP-531. AZP-531 significantly decreased post-prandial glucose levels in a baseline glucose dependent fashion.
Conclusions: AZP-531 may constitute a new treatment strategy to improve hyperphagia and metabolic issues in patients with PWS. These findings support further investigation in longer-term clinical trials.
Conflict of interest statement
Figures


References
-
- Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14(1):10–26. doi: 10.1038/gim.0b013e31822bead0 . - DOI - PubMed
-
- Miller JL, Lynn CH, Driscoll DC, Goldstone AP, Gold JA, Kimonis V, et al. Nutritional phases in Prader-Willi syndrome. Am J Med Genet A. 2011;155A(5):1040–9. doi: 10.1002/ajmg.a.33951 - DOI - PMC - PubMed
-
- Tauber M, Diene G, Mimoun E, Cabal-Berthoumieu S, Mantoulan C, Molinas C, et al. Prader-Willi syndrome as a model of human hyperphagia. Front Horm Res. 2014;42:93–106. doi: 10.1159/000358317 . - DOI - PubMed
-
- Lopez-Bastida J, Linertova R, Oliva-Moreno J, Posada-de-la-Paz M, Serrano-Aguilar P, Kanavos P, et al. Social/economic costs and health-related quality of life in patients with Prader-Willi syndrome in Europe. Eur J Health Econ. 2016;17 Suppl 1:99–108. doi: 10.1007/s10198-016-0788-z - DOI - PubMed
-
- Chevreul K, Berg BK, Clement MC, Poitou C, Tauber M. Economic burden and health-related quality of life associated with Prader-Willi syndrome in France. J Intellect Disabil Res. 2016;60(9):879–90. doi: 10.1111/jir.12288 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

