The potent effect of mycolactone on lipid membranes
- PMID: 29320578
- PMCID: PMC5779694
- DOI: 10.1371/journal.ppat.1006814
The potent effect of mycolactone on lipid membranes
Abstract
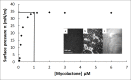
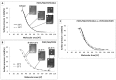
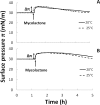
Mycolactone is a lipid-like endotoxin synthesized by an environmental human pathogen, Mycobacterium ulcerans, the causal agent of Buruli ulcer disease. Mycolactone has pleiotropic effects on fundamental cellular processes (cell adhesion, cell death and inflammation). Various cellular targets of mycolactone have been identified and a literature survey revealed that most of these targets are membrane receptors residing in ordered plasma membrane nanodomains, within which their functionalities can be modulated. We investigated the capacity of mycolactone to interact with membranes, to evaluate its effects on membrane lipid organization following its diffusion across the cell membrane. We used Langmuir monolayers as a cell membrane model. Experiments were carried out with a lipid composition chosen to be as similar as possible to that of the plasma membrane. Mycolactone, which has surfactant properties, with an apparent saturation concentration of 1 μM, interacted with the membrane at very low concentrations (60 nM). The interaction of mycolactone with the membrane was mediated by the presence of cholesterol and, like detergents, mycolactone reshaped the membrane. In its monomeric form, this toxin modifies lipid segregation in the monolayer, strongly affecting the formation of ordered microdomains. These findings suggest that mycolactone disturbs lipid organization in the biological membranes it crosses, with potential effects on cell functions and signaling pathways. Microdomain remodeling may therefore underlie molecular events, accounting for the ability of mycolactone to attack multiple targets and providing new insight into a single unifying mechanism underlying the pleiotropic effects of this molecule. This membrane remodeling may act in synergy with the other known effects of mycolactone on its intracellular targets, potentiating these effects.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Simpson C, O'Brien DP, McDonald A, Callan P. Mycobacterium ulcerans infection: evolution in clinical management. ANZ J Surg. 2013; 83: 523–526. doi: 10.1111/j.1445-2197.2012.06230.x - DOI - PubMed
-
- Chany A-C, Tresse C, Casarotto V, Blanchard N. History, biology and chemistry of Mycobacterium ulcerans infections (Buruli ulcer disease). Nat Prod Rep. 2013; 30: 1527–1567. doi: 10.1039/c3np70068b - DOI - PubMed
-
- Bratschi MW, Ruf M-T, Andreoli A, Minyem JC, Kerber S, Wantong FG, et al. Mycobacterium ulcerans persistence at a village water source of Buruli ulcer patients. PLoS Negl Trop Dis. 2014; 8: e2756 doi: 10.1371/journal.pntd.0002756 - DOI - PMC - PubMed
-
- Walsh DS, Portaels F, Meyers WM. Buruli ulcer: advances in understanding Mycobacterium ulcerans infection. Dermatol Clin. 2011; 29: 1–8. doi: 10.1016/j.det.2010.09.006 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

