Detection of fast oscillating magnetic fields using dynamic multiple TR imaging and Fourier analysis
- PMID: 29320580
- PMCID: PMC5761850
- DOI: 10.1371/journal.pone.0189916
Detection of fast oscillating magnetic fields using dynamic multiple TR imaging and Fourier analysis
Abstract
Neuronal oscillations produce oscillating magnetic fields. There have been trials to detect neuronal oscillations using MRI, but the detectability in in vivo is still in debate. Major obstacles to detecting neuronal oscillations are (i) weak amplitudes, (ii) fast oscillations, which are faster than MRI temporal resolution, and (iii) random frequencies and on/off intervals. In this study, we proposed a new approach for direct detection of weak and fast oscillating magnetic fields. The approach consists of (i) dynamic acquisitions using multiple times to repeats (TRs) and (ii) an expanded frequency spectral analysis. Gradient echo echo-planar imaging was used to test the feasibility of the proposed approach with a phantom generating oscillating magnetic fields with various frequencies and amplitudes and random on/off intervals. The results showed that the proposed approach could precisely detect the weak and fast oscillating magnetic fields with random frequencies and on/off intervals. Complex and phase spectra showed reliable signals, while no meaningful signals were observed in magnitude spectra. A two-TR approach provided an absolute frequency spectrum above Nyquist sampling frequency pixel by pixel with no a priori target frequency information. The proposed dynamic multiple-TR imaging and Fourier analysis are promising for direct detection of neuronal oscillations and potentially applicable to any pulse sequences.
Conflict of interest statement
Figures

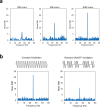
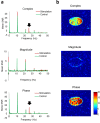


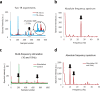
References
-
- Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64(3):803–12. doi: 10.1016/S0006-3495(93)81441-3 ; PubMed Central PMCID: PMCPMC1262394. - DOI - PMC - PubMed
-
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–94. doi: 10.1038/35094500 . - DOI - PubMed
-
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar Mri. Magnet Reson Med. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409 PubMed PMID: WOS:A1995RX75100008. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

