Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children
- PMID: 29320603
- PMCID: PMC6491279
- DOI: 10.1002/14651858.CD001905.pub3
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children
Abstract
Background: Tonic-clonic convulsions and convulsive status epilepticus (currently defined as a tonic-clonic convulsion lasting at least 30 minutes) are medical emergencies and require urgent and appropriate anticonvulsant treatment. International consensus is that an anticonvulsant drug should be administered for any tonic-clonic convulsion that has been continuing for at least five minutes. Benzodiazepines (diazepam, lorazepam, midazolam) are traditionally regarded as first-line drugs and phenobarbital, phenytoin and paraldehyde as second-line drugs. This is an update of a Cochrane Review first published in 2002 and updated in 2008.
Objectives: To evaluate the effectiveness and safety of anticonvulsant drugs used to treat any acute tonic-clonic convulsion of any duration, including established convulsive (tonic-clonic) status epilepticus in children who present to a hospital or emergency medical department.
Search methods: For the latest update we searched the Cochrane Epilepsy Group's Specialised Register (23 May 2017), the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO, 23 May 2017), MEDLINE (Ovid, 1946 to 23 May 2017), ClinicalTrials.gov (23 May 2017), and the WHO International Clinical Trials Registry Platform (ICTRP, 23 May 2017).
Selection criteria: Randomised and quasi-randomised trials comparing any anticonvulsant drugs used for the treatment of an acute tonic-clonic convulsion including convulsive status epilepticus in children.
Data collection and analysis: Two review authors independently assessed trials for inclusion and extracted data. We contacted study authors for additional information.
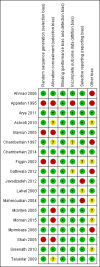
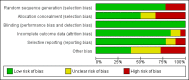
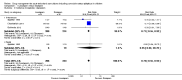
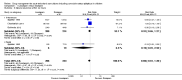
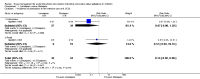
Main results: The review includes 18 randomised trials involving 2199 participants, and a range of drug treatment options, doses and routes of administration (rectal, buccal, nasal, intramuscular and intravenous). The studies vary by design, setting and population, both in terms of their ages and also in their clinical situation. We have made many comparisons of drugs and of routes of administration of drugs in this review; our key findings are as follows:(1) This review provides only low- to very low-quality evidence comparing buccal midazolam with rectal diazepam for the treatment of acute tonic-clonic convulsions (risk ratio (RR) for seizure cessation 1.25, 95% confidence interval (CI) 1.13 to 1.38; 4 trials; 690 children). However, there is uncertainty about the effect and therefore insufficient evidence to support its use. There were no included studies which compare intranasal and buccal midazolam.(2) Buccal and intranasal anticonvulsants were shown to lead to similar rates of seizure cessation as intravenous anticonvulsants, e.g. intranasal lorazepam appears to be as effective as intravenous lorazepam (RR 0.96, 95% CI 0.82 to 1.13; 1 trial; 141 children; high-quality evidence) and intranasal midazolam was equivalent to intravenous diazepam (RR 0.98, 95% CI 0.91 to 1.06; 2 trials; 122 children; moderate-quality evidence).(3) Intramuscular midazolam also showed a similar rate of seizure cessation to intravenous diazepam (RR 0.97, 95% CI 0.87 to 1.09; 2 trials; 105 children; low-quality evidence).(4) For intravenous routes of administration, lorazepam appears to be as effective as diazepam in stopping acute tonic clonic convulsions: RR 1.04, 95% CI 0.94 to 1.16; 3 trials; 414 children; low-quality evidence. Furthermore, we found no statistically significant or clinically important differences between intravenous midazolam and diazepam (RR for seizure cessation 1.08, 95% CI 0.97 to 1.21; 1 trial; 80 children; moderate-quality evidence) or intravenous midazolam and lorazepam (RR for seizure cessation 0.98, 95% CI 0.91 to 1.04; 1 trial; 80 children; moderate-quality evidence). In general, intravenously-administered anticonvulsants led to more rapid seizure cessation but this was usually compromised by the time taken to establish intravenous access.(5) There is limited evidence from a single trial to suggest that intranasal lorazepam may be more effective than intramuscular paraldehyde in stopping acute tonic-clonic convulsions (RR 1.22, 95% CI 0.99 to 1.52; 160 children; moderate-quality evidence).(6) Adverse side effects were observed and reported very infrequently in the included studies. Respiratory depression was the most common and most clinically relevant side effect and, where reported, the frequency of this adverse event was observed in 0% to up to 18% of children. None of the studies individually demonstrated any difference in the rates of respiratory depression between the different anticonvulsants or their different routes of administration; but when pooled, three studies (439 children) provided moderate-quality evidence that lorazepam was significantly associated with fewer occurrences of respiratory depression than diazepam (RR 0.72, 95% CI 0.55 to 0.93).Much of the evidence provided in this review is of mostly moderate to high quality. However, the quality of the evidence provided for some important outcomes is low to very low, particularly for comparisons of non-intravenous routes of drug administration. Low- to very low-quality evidence was provided where limited data and imprecise results were available for analysis, methodological inadequacies were present in some studies which may have introduced bias into the results, study settings were not applicable to wider clinical practice, and where inconsistency was present in some pooled analyses.
Authors' conclusions: We have not identified any new high-quality evidence on the efficacy or safety of an anticonvulsant in stopping an acute tonic-clonic convulsion that would inform clinical practice. There appears to be a very low risk of adverse events, specifically respiratory depression. Intravenous lorazepam and diazepam appear to be associated with similar rates of seizure cessation and respiratory depression. Although intravenous lorazepam and intravenous diazepam lead to more rapid seizure cessation, the time taken to obtain intravenous access may undermine this effect. In the absence of intravenous access, buccal midazolam or rectal diazepam are therefore acceptable first-line anticonvulsants for the treatment of an acute tonic-clonic convulsion that has lasted at least five minutes. There is no evidence provided by this review to support the use of intranasal midazolam or lorazepam as alternatives to buccal midazolam or rectal diazepam.
Conflict of interest statement
Richard Appleton is the lead investigator of the study included in the original review and is a co‐author of another study included in this update. Tim Martland is a co‐author of one of the studies included in this review. Amy McTague has no known declarations of interests.
Figures


































Update of
-
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001905. doi: 10.1002/14651858.CD001905.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2018 Jan 10;1:CD001905. doi: 10.1002/14651858.CD001905.pub3. PMID: 18646081 Updated.
Similar articles
-
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001905. doi: 10.1002/14651858.CD001905.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2018 Jan 10;1:CD001905. doi: 10.1002/14651858.CD001905.pub3. PMID: 18646081 Updated.
-
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children.Cochrane Database Syst Rev. 2002;(4):CD001905. doi: 10.1002/14651858.CD001905. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001905. doi: 10.1002/14651858.CD001905.pub2. PMID: 12519562 Updated.
-
Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2017 Feb 27;2(2):CD001911. doi: 10.1002/14651858.CD001911.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Jul 18;7:CD001911. doi: 10.1002/14651858.CD001911.pub4. PMID: 28240353 Free PMC article. Updated.
-
Lamotrigine versus carbamazepine monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2018 Jun 28;6(6):CD001031. doi: 10.1002/14651858.CD001031.pub4. Cochrane Database Syst Rev. 2018. PMID: 29952431 Free PMC article.
-
Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2015 Aug 14;(8):CD001911. doi: 10.1002/14651858.CD001911.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2017 Feb 27;2:CD001911. doi: 10.1002/14651858.CD001911.pub3. PMID: 26275105 Updated.
Cited by
-
Treatment of pediatric convulsive status epilepticus.Front Neurol. 2023 Jun 29;14:1175370. doi: 10.3389/fneur.2023.1175370. eCollection 2023. Front Neurol. 2023. PMID: 37456627 Free PMC article. Review.
-
[Paediatric Life Support].Notf Rett Med. 2021;24(4):650-719. doi: 10.1007/s10049-021-00887-9. Epub 2021 Jun 2. Notf Rett Med. 2021. PMID: 34093080 Free PMC article. Review. German.
-
Febrile seizures: an overview.Drugs Context. 2018 Jul 16;7:212536. doi: 10.7573/dic.212536. eCollection 2018. Drugs Context. 2018. PMID: 30038660 Free PMC article. Review.
-
Pharmacotherapy for Focal Seizures in Children and Adolescents.Drugs. 2018 Sep;78(13):1321-1337. doi: 10.1007/s40265-018-0959-6. Drugs. 2018. PMID: 30128698 Review.
-
Family Game Show-style Didactic for Teaching Nervous System Disorders during Emergency Medicine Training.J Educ Teach Emerg Med. 2020 Apr 15;5(2):L1-L19. doi: 10.21980/J8D357. eCollection 2020 Apr. J Educ Teach Emerg Med. 2020. PMID: 37465401 Free PMC article.
References
References to studies included in this review
Ahmad 2006 {published data only}
-
- Ahmad S, Ellis JC, Kamwendo H, Molyneux E. Efficacy and safety of intranasal lorazepam versus intramuscular paraldehyde for protracted convulsions in children: an open randomised trial. Lancet 2006;367(9522):1591‐7. - PubMed
-
- NCT00116064. Intranasal lorazepam versus intramuscular paraldehyde in paediatric convulsions. ClinicalTrials.gov/show/NCT00116064 (first received July 2004).
Appleton 1995 {published data only}
-
- Appleton RE, Sweeney A, Choonara I, Robson J, Molyneux E. Lorazepam versus diazepam in the acute treatment of epileptic seizures and status epilepticus. Developmental Medicine and Child Neurology 1995;37:682‐8. - PubMed
Arya 2011 {published data only}
-
- Arya R, Gulati S, Kabra M, Sahu J, Kalra V. Intranasal versus intravenous lorazepam for control of acute seizures in children: a randomized open‐label study. Epilepsia 2011;52(4):788‐93. - PubMed
Ashrafi 2010 {published data only}
-
- Ashrafi M, Khosroshahi N, Karimi P, Malamiri R, Bavarian B, Zarch A, et al. Efficacy and usability of buccal midazolam in controlling acute prolonged convulsive seizures in children. European Journal of Paediatric Neurology 2010;14(5):434‐8. - PubMed
Baysun 2005 {published data only}
-
- Baysun S, Aydin OF, Atmaca E, Gurer Y. A comparison of buccal midazolam and rectal diazepam for the acute treatment of seizures. Clinical Pediatrics 2005;44(9):771‐6. - PubMed
Chamberlain 1997 {published and unpublished data}
-
- Chamberlain JM, Altieri MA, Futterman C, Young GM, Ochsenschlager DW, Waisman Y. A prospective, randomized study comparing intramuscular midazolam with intravenous diazepam for the treatment of seizures in children. Pediatric Emergency Care 1997; Vol. 13, issue 2:92‐4. - PubMed
Chamberlain 2014 {published data only}
-
- Chamberlain JM, Okada P, Holsti M, Mahajan P, Brown KM, Vance C, et al. Lorazepam vs diazepam for pediatric status epilepticus: a randomized clinical trial. JAMA 2014;311(16):1652‐60. - PubMed
Fişgin 2002 {published data only}
-
- Fişgin T, Gurer Y, Teziç T, Senbil N, Zorlu P, Okuyaz C, et al. Effects of intranasal midazolam and rectal diazepam on acute convulsions in children: prospective randomized study. Journal of Child Neurology 2002;17(2):123‐6. - PubMed
Gathwala 2012 {published data only}
-
- Gathwala G, Goel M, Singh J, Mittal K. Intravenous diazepam, midazolam and lorazepam in acute seizure control. Indian Journal of Pediatrics 2012;79(3):327‐32. - PubMed
Javadzadeh 2012 {published data only}
Lahat 2000 {published data only}
Mahmoudian 2004 {published data only}
-
- Mahmoudian T, Mohammadi Zadeh M. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy and Behavior 2004;5:253‐5. - PubMed
McIntyre 2005 {published data only}
-
- Appleton RE, McIntyre JW, Choonara IA, Whitehouse WP, Robertson S, Norris E. Randomised controlled trial of buccal midazolam versus rectal diazepam for the emergency treatment of seizures in children. Epilepsia 2004;45(Suppl 7):186, Abstract no: B.02. - PubMed
-
- McIntyre J, Robertson S, Norris E, Appleton RE, Whitehouse WP, Phillips B, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet 2005;366(9481):205‐10. - PubMed
Momen 2015 {published data only}
-
- Momen AA, Azizi Malamiri R, Nikkhah A, Jafari M, Fayezi A, Riahi K, Maraghi E. Efficacy and safety of intramuscular midazolam versus rectal diazepam in controlling status epilepticus in children.. European Journal of Paediatric Neurology: EJPN 2015;19(2):149‐54. - PubMed
Mpimbaza 2008 {published data only}
-
- Mpimbaza A, Ndeezi G, Staedke S, Rosenthal PJ, Byarugaba J. Comparison of buccal midazolam with rectal diazepam in the treatment of prolonged seizures in Ugandan children: a randomized clinical trial. Pediatrics 2008;121(1):e58‐64. [PUBMED: 18166545] - PubMed
Shah 2005 {published data only}
-
- Shah I, Deshmukh CT. Intramuscular midazolam vs intravenous diazepam for acute seizures. Indian Journal of Pediatrics 2005; Vol. 72, issue 8:667‐70. - PubMed
Sreenath 2010 {published and unpublished data}
-
- Sreenath TG, Gupta P, Sharma KK, Krishnamurthy S. Lorazepam versus diazepam‐phenytoin combination in the treatment of convulsive status epilepticus in children: a randomized controlled trial. European Journal of Paediatric Neurology 2010;14(2):162‐8. - PubMed
Talukdar 2009 {published data only}
-
- Talukdar B, Chakrabarty B. Efficacy of buccal midazolam compared to intravenous diazepam in controlling convulsions in children: a randomised controlled trial. Brain and Development 2009;31(10):744‐9. - PubMed
References to studies excluded from this review
Agarwal 2007 {published data only}
-
- Agarwal P, Kumar N, Chandra R, Gupta G, Antony AR, Garg N. Randomized study of intravenous valproate and phenytoin in status epilepticus. Seizure 2007;16(6):527‐32. - PubMed
Arpita 2014 {published data only}
-
- Arpita A, Chandrakanta, Kumar R, Singh SN. Efficacy of intravenous valproate versus intravenous phenytoin in children with status epilepticus: a randomized controlled trial in tertiary care centre. Pediatric Critical Care Medicine 2014;15(4 Suppl 1):11, Abstract no: 31. [DOI: ]
Bhattacharyya 2006 {published data only}
-
- Bhattacharyya M, Kalra V, Gulati S. Intranasal midazolam vs rectal diazepam in acute childhood seizures. Pediatric Neurology 2006;34(5):355‐9. - PubMed
Camfield 1980 {published data only}
-
- Camfield PR, Camfield CS, Shapiro SH, Cummings C. The first febrile seizure‐‐antipyretic instruction plus either phenobarbital or placebo to prevent recurrence. Journal of Pediatrics 1980;97(1):16‐21. - PubMed
Cereghino 1998 {published data only}
-
- Cereghino JJ, Mitchell WG, Murphy J, Kriel RL, Rosenfeld WE, Trevathan E. Treating repetitive seizures with a rectal diazepam formulation: a randomized study. The North American Diastat Study Group. Neurology 1998;51(5):1274‐82. - PubMed
Heckmatt 1976 {published data only}
Holsti 2010 {published data only}
-
- Holsti M, Dudley N, Schunk J, Adelgais K, Greenberg R, Olsen C, et al. Intranasal midazolam vs rectal diazepam for the home treatment of acute seizures in pediatric patients with epilepsy. Archives of Pediatrics and Adolescent Medicine 2010;164(8):747‐53. - PubMed
Kutlu 2003 {published data only}
-
- Kutlu NO, Dogrul M, Yakinci C, Soylu H. Buccal midazolam for treatment of prolonged seizures. Brain and Development 2003;25(4):275‐8. - PubMed
Mahmoudian 2006 {published data only}
-
- Mahmoudian T, Najafian M. Comparing the effects of intravenous midazolam with rectal sodium valproate in controlling of children with refractory status epilepticus. Journal of Research in Medical Science 2006;11(1):1‐5.
McCormick 1999 {published data only}
-
- McCormick EM, Lieh‐Lai M, Knazik S, Nigro M. A prospective comparison of midazolam and lorazepam in the initial treatment of status epilepticus in the pediatric patient. Epilepsia 1999; Vol. 40 Suppl 7:160.
Mehta 2007 {published data only}
-
- Mehta V, Singhi P, Singhi S. Intravenous sodium valproate versus diazepam for the control of refractory status epilepticus in children: a randomised controlled trial. Journal of Child Neurology 2007;22(10):1191‐7. - PubMed
Mittal 2014 {published data only}
-
- Mittal K, Gupta A. Efficacy & safety: Intravenous sodium valproate and phenytoin in status epilepticus. Critical Care Medicine 2014;42(12 Suppl 1):A1486‐A7. [DOI: 10.1097/01.ccm.0000458023.53658.51] - DOI
Morton 2007 {published data only}
-
- Morton LD, O'Hara KA, Coots BP, Pellock JM. Safety of rapid intravenous valproate infusion in pediatric patients. Pediatric Neurology 2007; Vol. 36, issue 2:81‐3. - PubMed
Qureshi 2002 {published data only}
-
- Qureshi A, Wassmer E, Davies P, Berry K, Whitehouse W. Comparative audit of intravenous lorazepam and diazepam in the emergency treatment of convulsive status epilepticus in children. Seizure 2002;11(3):141‐4. - PubMed
Rosati 2016 {published data only}
-
- Rosati A, Ilvento L, L'Erario M, Masi S, Biggeri A, Fabbro G, et al. Efficacy of ketamine in refractory convulsive status epilepticus in children: a protocol for a sequential design, multicentre, randomised, controlled, open‐label, non‐profit trial (KETASER01). BMJ Open 2016;6(6):e011565. [DOI: 10.1136/bmjopen-2016-011565.; PUBMED: 27311915] - DOI - PMC - PubMed
Scott 1999 {published data only}
-
- Scott RC, Besag FM, Neville BG. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet 1999;353(9153):623‐6. - PubMed
Silbergleit 2012 {published data only}
Singhi 2002 {published data only}
-
- Singhi S, Murthy A, Singhi P, Jayashree M. Continuous midazolam versus diazepam for refractory convulsive status epilepticus. Journal of Child Neurology 2002;17(2):106‐10. - PubMed
Strengell 2009 {published data only}
-
- Strengell T, Uhari M, Tarkka R, Uusimaa J, Alen R, Lautala P, et al. Antipyretic agents for preventing recurrences of febrile seizures: randomized controlled trial. Archives of Pediatrics & Adolescent Medicine 2009;163(9):799‐804. - PubMed
Tonekaboni 2012 {published data only (unpublished sought but not used)}
Additional references
APLS 2016
-
- Advanced Life Support Group. Advanced Paediatric Life Support (APLS): A Practical Approach to Emergencies. 6th Edition. New Jersey (USA): Wiley‐Blackwell Publishing, 2016.
Chin 2008
Garr 1999
-
- Garr RE, Appleton RE, Robson WJ, Molyneux EM. Children presenting with convulsions (including status epilepticus) to a paediatric accident and emergency department: an audit of a treatment protocol. Developmental Medicine and Child Neurology 1999;41(1):44‐7. - PubMed
Glauser 2016
GRADEPro 2004 [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEPro Version 3.6 for Windows. GRADE Working Group, 2004.
Higgins 2011a
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
McMullan 2010
MECIR 2012
-
- Methodological Expectations of Cochrane Intervention Reviews (MECIR): Standards for the conduct and reporting of new Cochrane Intervention Reviews 2012. editorial‐unit.cochrane.org.
NICE 2012
-
- National Institute for Health and Care Excellence. Epilepsies: diagnosis and management. Available from www.nice.org.uk/guidance/cg137 Accessed 1st July 2017.
Rowland 2009
-
- Rowland AG, Gill AM, Stewart AB, Appleton RE, Al Kharusi A, Cramp C, et al. Review of the efficacy of rectal paraldehyde in the management of acute and prolonged tonic–clonic convulsions. Archives of Disease in Childhood 2009;94(9):720‐3. - PubMed
Shinnar 2001
-
- Shinnar S, Berg AT. How long do new‐onset seizures in children last?. Annals of Neurology 2001;49(5):659‐64. - PubMed
Silbergleit 2013
Trinka 2015
-
- Trinka E, Cock H, Hesdorffer D. A definition and classification of status epilepticus – Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015;56(10):1515‐23. - PubMed
Welch 2015
-
- Welch RD, Nicholas K, Durkalski‐Mauldin VL, Lowenstein DH, Conwit R, Mahajan PV, et al. Neurological Emergencies Treatment Trials (NETT) Network Investigators. Intramuscular midazolam versus intravenous lorazepam for the prehospital treatment of status epilepticus in the pediatric population.. Epilepsia 2015;56(2):254‐62. - PMC - PubMed
References to other published versions of this review
Appleton 2000
Appleton 2007
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

