Essential Role of the ε Subunit for Reversible Chemo-Mechanical Coupling in F1-ATPase
- PMID: 29320685
- PMCID: PMC5773760
- DOI: 10.1016/j.bpj.2017.11.004
Essential Role of the ε Subunit for Reversible Chemo-Mechanical Coupling in F1-ATPase
Abstract
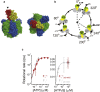
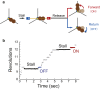
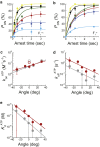
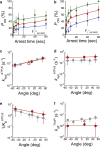
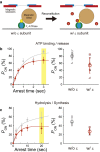
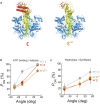
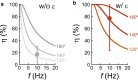
F1-ATPase is a rotary motor protein driven by ATP hydrolysis. Among molecular motors, F1 exhibits unique high reversibility in chemo-mechanical coupling, synthesizing ATP from ADP and inorganic phosphate upon forcible rotor reversal. The ε subunit enhances ATP synthesis coupling efficiency to > 70% upon rotation reversal. However, the detailed mechanism has remained elusive. In this study, we performed stall-and-release experiments to elucidate how the ε subunit modulates ATP association/dissociation and hydrolysis/synthesis process kinetics and thermodynamics, key reaction steps for efficient ATP synthesis. The ε subunit significantly accelerated the rates of ATP dissociation and synthesis by two- to fivefold, whereas those of ATP binding and hydrolysis were not enhanced. Numerical analysis based on the determined kinetic parameters quantitatively reproduced previous findings of two- to fivefold coupling efficiency improvement by the ε subunit at the condition exhibiting the maximum ATP synthesis activity, a physiological role of F1-ATPase. Furthermore, fundamentally similar results were obtained upon ε subunit C-terminal domain truncation, suggesting that the N-terminal domain is responsible for the rate enhancement.
Copyright © 2017 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
F1-ATPase: a highly coupled reversible rotary motor.Biochem Soc Trans. 2006 Nov;34(Pt 5):993-6. doi: 10.1042/BST0340993. Biochem Soc Trans. 2006. PMID: 17052244
-
Chemomechanical coupling mechanism of F(1)-ATPase: catalysis and torque generation.FEBS Lett. 2013 Apr 17;587(8):1030-5. doi: 10.1016/j.febslet.2013.01.063. Epub 2013 Feb 8. FEBS Lett. 2013. PMID: 23395605 Review.
-
A systematic assessment of mycobacterial F1 -ATPase subunit ε's role in latent ATPase hydrolysis.FEBS J. 2021 Feb;288(3):818-836. doi: 10.1111/febs.15440. Epub 2020 Jul 4. FEBS J. 2021. PMID: 32525613
-
The regulator of the F1 motor: inhibition of rotation of cyanobacterial F1-ATPase by the epsilon subunit.EMBO J. 2006 Oct 4;25(19):4596-604. doi: 10.1038/sj.emboj.7601348. Epub 2006 Sep 14. EMBO J. 2006. PMID: 16977308 Free PMC article.
-
ATP synthase from Escherichia coli: Mechanism of rotational catalysis, and inhibition with the ε subunit and phytopolyphenols.Biochim Biophys Acta. 2016 Feb;1857(2):129-140. doi: 10.1016/j.bbabio.2015.11.005. Epub 2015 Nov 14. Biochim Biophys Acta. 2016. PMID: 26589785 Review.
Cited by
-
The regulatory subunit ε in Escherichia coli FOF1-ATP synthase.Biochim Biophys Acta Bioenerg. 2018 Sep;1859(9):775-788. doi: 10.1016/j.bbabio.2018.06.013. Epub 2018 Jun 20. Biochim Biophys Acta Bioenerg. 2018. PMID: 29932911 Free PMC article. Review.
-
F1-ATPase Rotary Mechanism: Interpreting Results of Diverse Experimental Modes With an Elastic Coupling Theory.Front Microbiol. 2022 Apr 22;13:861855. doi: 10.3389/fmicb.2022.861855. eCollection 2022. Front Microbiol. 2022. PMID: 35531282 Free PMC article. Review.
-
Rotary properties of hybrid F1-ATPases consisting of subunits from different species.iScience. 2023 Apr 8;26(5):106626. doi: 10.1016/j.isci.2023.106626. eCollection 2023 May 19. iScience. 2023. PMID: 37192978 Free PMC article.
References
-
- Yoshida M., Muneyuki E., Hisabori T. ATP synthase--a marvellous rotary engine of the cell. Nat. Rev. Mol. Cell Biol. 2001;2:669–677. - PubMed
-
- Junge W., Sielaff H., Engelbrecht S. Torque generation and elastic power transmission in the rotary F(O)F(1)-ATPase. Nature. 2009;459:364–370. - PubMed
-
- Weber J. Structural biology: toward the ATP synthase mechanism. Nat. Chem. Biol. 2010;6:794–795. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

