Collective Cell Migration in Embryogenesis Follows the Laws of Wetting
- PMID: 29320689
- PMCID: PMC5773767
- DOI: 10.1016/j.bpj.2017.11.011
Collective Cell Migration in Embryogenesis Follows the Laws of Wetting
Abstract
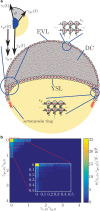
Collective cell migration is a fundamental process during embryogenesis and its initial occurrence, called epiboly, is an excellent in vivo model to study the physical processes involved in collective cell movements that are key to understanding organ formation, cancer invasion, and wound healing. In zebrafish, epiboly starts with a cluster of cells at one pole of the spherical embryo. These cells are actively spreading in a continuous movement toward its other pole until they fully cover the yolk. Inspired by the physics of wetting, we determine the contact angle between the cells and the yolk during epiboly. By choosing a wetting approach, the relevant scale for this investigation is the tissue level, which is in contrast to other recent work. Similar to the case of a liquid drop on a surface, one observes three interfaces that carry mechanical tension. Assuming that interfacial force balance holds during the quasi-static spreading process, we employ the physics of wetting to predict the temporal change of the contact angle. Although the experimental values vary dramatically, the model allows us to rescale all measured contact-angle dynamics onto a single master curve explaining the collective cell movement. Thus, we describe the fundamental and complex developmental mechanism at the onset of embryogenesis by only three main parameters: the offset tension strength, α, that gives the strength of interfacial tension compared to other force-generating mechanisms; the tension ratio, δ, between the different interfaces; and the rate of tension variation, λ, which determines the timescale of the whole process.
Copyright © 2017 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Surfactant solutions and porous substrates: spreading and imbibition.Adv Colloid Interface Sci. 2004 Nov 29;111(1-2):3-27. doi: 10.1016/j.cis.2004.07.007. Adv Colloid Interface Sci. 2004. PMID: 15571660
-
Review of non-reactive and reactive wetting of liquids on surfaces.Adv Colloid Interface Sci. 2007 Jun 30;133(2):61-89. doi: 10.1016/j.cis.2007.04.009. Epub 2007 May 1. Adv Colloid Interface Sci. 2007. PMID: 17560842
-
Spreading of liquid drops over porous substrates.Adv Colloid Interface Sci. 2003 Jul 1;104:123-58. doi: 10.1016/s0001-8686(03)00039-3. Adv Colloid Interface Sci. 2003. PMID: 12818493
-
Active wetting of epithelial tissues: modeling considerations.Eur Biophys J. 2023 Feb;52(1-2):1-15. doi: 10.1007/s00249-022-01625-w. Epub 2023 Jan 2. Eur Biophys J. 2023. PMID: 36593348 Review.
-
Physics of collective cell migration.Eur Biophys J. 2023 Nov;52(8):625-640. doi: 10.1007/s00249-023-01681-w. Epub 2023 Sep 14. Eur Biophys J. 2023. PMID: 37707627 Review.
Cited by
-
Global and local tension measurements in biomimetic skeletal muscle tissues reveals early mechanical homeostasis.Elife. 2021 Jan 18;10:e60145. doi: 10.7554/eLife.60145. Elife. 2021. PMID: 33459593 Free PMC article.
-
Compressive stress triggers fibroblasts spreading over cancer cells to generate carcinoma in situ organization.Commun Biol. 2024 Feb 15;7(1):184. doi: 10.1038/s42003-024-05883-6. Commun Biol. 2024. PMID: 38360973 Free PMC article.
-
Tissue rheology in embryonic organization.EMBO J. 2019 Oct 15;38(20):e102497. doi: 10.15252/embj.2019102497. Epub 2019 Sep 12. EMBO J. 2019. PMID: 31512749 Free PMC article. Review.
-
Interplay among cell migration, shaping, and traction force on a matrix with cell-scale stiffness heterogeneity.Biophys Physicobiol. 2022 Sep 13;19:e190036. doi: 10.2142/biophysico.bppb-v19.0036. eCollection 2022. Biophys Physicobiol. 2022. PMID: 36349327 Free PMC article.
-
The Dynamic Counterbalance of RAC1-YAP/OB-Cadherin Coordinates Tissue Spreading with Stem Cell Fate Patterning.Adv Sci (Weinh). 2021 Mar 8;8(10):2004000. doi: 10.1002/advs.202004000. eCollection 2021 May. Adv Sci (Weinh). 2021. PMID: 34026448 Free PMC article.
References
-
- Kimmel C.B., Ballard W.W., Schilling T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. - PubMed
-
- Warga R.M., Kimmel C.B. Cell movements during epiboly and gastrulation in zebrafish. Development. 1990;108:569–580. - PubMed
-
- Kimmel C.B., Law R.D. Cell lineage of zebrafish blastomeres. II. Formation of the yolk syncytial layer. Dev. Biol. 1985;108:86–93. - PubMed
-
- Carvalho L., Heisenberg C.-P. The yolk syncytial layer in early zebrafish development. Trends Cell Biol. 2010;20:586–592. - PubMed
-
- Weliky M., Oster G. The mechanical basis of cell rearrangement. I. Epithelial morphogenesis during Fundulus epiboly. Development. 1990;109:373–386. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

