Esrrb Complementation Rescues Development of Nanog-Null Germ Cells
- PMID: 29320730
- PMCID: PMC5775501
- DOI: 10.1016/j.celrep.2017.12.060
Esrrb Complementation Rescues Development of Nanog-Null Germ Cells
Abstract
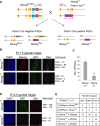
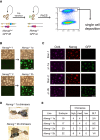
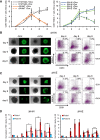
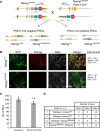
The transcription factors (TFs) Nanog and Esrrb play important roles in embryonic stem cells (ESCs) and during primordial germ-cell (PGC) development. Esrrb is a positively regulated direct target of NANOG in ESCs that can substitute qualitatively for Nanog function in ESCs. Whether this functional substitution extends to the germline is unknown. Here, we show that germline deletion of Nanog reduces PGC numbers 5-fold at midgestation. Despite this quantitative depletion, Nanog-null PGCs can complete germline development in contrast to previous findings. PGC-like cell (PGCLC) differentiation of Nanog-null ESCs is also impaired, with Nanog-null PGCLCs showing decreased proliferation and increased apoptosis. However, induced expression of Esrrb restores PGCLC numbers as efficiently as Nanog. These effects are recapitulated in vivo: knockin of Esrrb to Nanog restores PGC numbers to wild-type levels and results in fertile adult mice. These findings demonstrate that Esrrb can replace Nanog function in germ cells.
Keywords: PGCLCs; competence; naive pluripotency; primordial germ cells; transcription factors.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Beddington R.S. An autoradiographic analysis of tissue potency in different regions of the embryonic ectoderm during gastrulation in the mouse. J. Embryol. Exp. Morphol. 1982;69:265–285. - PubMed
-
- Campolo F., Gori M., Favaro R., Nicolis S., Pellegrini M., Botti F., Rossi P., Jannini E.A., Dolci S. Essential role of Sox2 for the establishment and maintenance of the germ cell line. Stem Cells. 2013;31:1408–1421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

