Development of Monoclonal Antibodies for Diagnosis of Plasmodium vivax
- PMID: 29320817
- PMCID: PMC5776898
- DOI: 10.3347/kjp.2017.55.6.623
Development of Monoclonal Antibodies for Diagnosis of Plasmodium vivax
Abstract
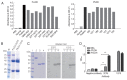
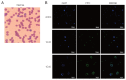
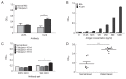
Plasmodium lactate dehydrogenase (pLDH) is a strong target antigen for the determination of infection with Plasmodium species specifically. However, a more effective antibody is needed because of the low sensitivity of the current antibody in many immunological diagnostic assays. In this study, recombinant Plasmodium vivax LDH (PvLDH) was experimentally constructed and expressed as a native antigen to develop an effective P. vivax-specific monoclonal antibody (mAb). Two mAbs (2CF5 and 1G10) were tested using ELISA and immunofluorescence assays (IFA), as both demonstrated reactivity against pLDH antigen. Of the 2 antibodies, 2CF5 was not able to detect P. falciparum, suggesting that it might possess P. vivax-specificity. The detection limit for a pair of 2 mAbs-linked sandwich ELISA was 31.3 ng/ml of the recombinant antigen. The P. vivax-specific performance of mAbs-linked ELISA was confirmed by in vitro-cultured P. falciparum and P. vivax-infected patient blood samples. In conclusion, the 2 new antibodies possessed the potential to detect P. vivax and will be useful in immunoassay.
Keywords: Plasmodium lactate dehydrogenase; Plasmodium vivax; monoclonal antibody.
Conflict of interest statement
All authors declare no conflict of interest.
Figures

Similar articles
-
A novel polyclonal antibody-based sandwich ELISA for detection of Plasmodium vivax developed from two lactate dehydrogenase protein segments.BMC Infect Dis. 2014 Jan 30;14:49. doi: 10.1186/1471-2334-14-49. BMC Infect Dis. 2014. PMID: 24475751 Free PMC article.
-
Production of monoclonal antibodies for Plasmodium vivax lactate dehydrogenase and patient sera screening using sandwich ELISA.Parasitol Res. 2012 Oct;111(4):1645-50. doi: 10.1007/s00436-012-3003-x. Epub 2012 Jun 28. Parasitol Res. 2012. PMID: 22740294
-
Performance of coumarin-derived dendrimer-based fluorescence-linked immunosorbent assay (FLISA) to detect malaria antigen.Malar J. 2014 Jul 10;13:266. doi: 10.1186/1475-2875-13-266. Malar J. 2014. PMID: 25011624 Free PMC article.
-
Two-site sandwich ELISA for detection of Plasmodium vivax blood stage antigens using monoclonal and polyclonal antibodies.Southeast Asian J Trop Med Public Health. 1992 Dec;23(4):745-51. Southeast Asian J Trop Med Public Health. 1992. PMID: 1298084
-
Soluble recombinant merozoite surface antigen-142kDa of Plasmodium vivax: An improved diagnostic antigen for vivax malaria.J Microbiol Methods. 2016 Apr;123:44-50. doi: 10.1016/j.mimet.2016.02.003. Epub 2016 Feb 3. J Microbiol Methods. 2016. PMID: 26851675
Cited by
-
Diagnostic Methods for Non-Falciparum Malaria.Front Cell Infect Microbiol. 2021 Jun 17;11:681063. doi: 10.3389/fcimb.2021.681063. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34222049 Free PMC article. Review.
-
Plasmodium falciparum Parasitemia and Band Sensitivity of the SD Bioline Malaria Ag P.f/Pan Rapid Diagnostic Test in Madagascar.Am J Trop Med Hyg. 2019 May;100(5):1196-1201. doi: 10.4269/ajtmh.18-1013. Am J Trop Med Hyg. 2019. PMID: 30834883 Free PMC article.
References
-
- Centers for Disease Control and Prevention Malaria [Internet] 2017 Available from: https://www.cdc.gov/malaria/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

