High-CO2 Requirement as a Mechanism for the Containment of Genetically Modified Cyanobacteria
- PMID: 29320853
- PMCID: PMC5793877
- DOI: 10.1021/acssynbio.7b00377
High-CO2 Requirement as a Mechanism for the Containment of Genetically Modified Cyanobacteria
Abstract
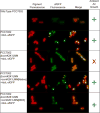
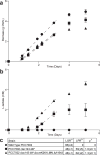
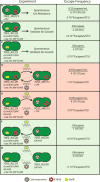
As researchers engineer cyanobacteria for biotechnological applications, we must consider potential environmental release of these organisms. Previous theoretical work has considered cyanobacterial containment through elimination of the CO2-concentrating mechanism (CCM) to impose a high-CO2 requirement (HCR), which could be provided in the cultivation environment but not in the surroundings. In this work, we experimentally implemented an HCR containment mechanism in Synechococcus sp. strain PCC7002 (PCC7002) through deletion of carboxysome shell proteins and showed that this mechanism contained cyanobacteria in a 5% CO2 environment. We considered escape through horizontal gene transfer (HGT) and reduced the risk of HGT escape by deleting competence genes. We showed that the HCR containment mechanism did not negatively impact the performance of a strain of PCC7002 engineered for L-lactate production. We showed through coculture experiments of HCR strains with ccm-containing strains that this HCR mechanism reduced the frequency of escape below the NIH recommended limit for recombinant organisms of one escape event in 108 CFU.
Keywords: CO2-concentrating mechanism; biocontainment; carboxysome; cyanobacteria; horizontal gene transfer; natural competence.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Biocontainment of Engineered Synechococcus elongatus PCC 7942 for Photosynthetic Production of α-Farnesene from CO2.J Agric Food Chem. 2021 Jan 20;69(2):698-703. doi: 10.1021/acs.jafc.0c07020. Epub 2021 Jan 7. J Agric Food Chem. 2021. PMID: 33411536
-
The CO2-concentrating mechanism of Synechococcus WH5701 is composed of native and horizontally-acquired components.Photosynth Res. 2011 Sep;109(1-3):59-72. doi: 10.1007/s11120-011-9641-5. Epub 2011 Mar 8. Photosynth Res. 2011. PMID: 21384181
-
Assessment of horizontal gene transfer-mediated destabilization of Synechococcus elongatus PCC 7942 biocontainment system.J Biosci Bioeng. 2023 Mar;135(3):190-195. doi: 10.1016/j.jbiosc.2022.12.002. Epub 2023 Jan 16. J Biosci Bioeng. 2023. PMID: 36653270
-
The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism.J Exp Bot. 2006;57(2):249-65. doi: 10.1093/jxb/eri286. Epub 2005 Oct 10. J Exp Bot. 2006. PMID: 16216846 Review.
-
[Progress in structure and CO2-concentrating mechanism of carboxysomes].Sheng Wu Gong Cheng Xue Bao. 2014 Aug;30(8):1164-71. Sheng Wu Gong Cheng Xue Bao. 2014. PMID: 25423746 Review. Chinese.
Cited by
-
Survivability of Wild-Type and Genetically Engineered Thermosynechococcus elongatus BP1 with Different Temperature Conditions.Appl Biosaf. 2020 Jun 1;25(2):104-117. doi: 10.1177/1535676019896640. Epub 2020 Jun 1. Appl Biosaf. 2020. PMID: 36035080 Free PMC article.
-
Microalgae for bioremediation: advances, challenges, and public perception on genetic engineering.BMC Plant Biol. 2024 Dec 27;24(1):1261. doi: 10.1186/s12870-024-05995-5. BMC Plant Biol. 2024. PMID: 39731038 Free PMC article. Review.
-
Controlling the Implementation of Transgenic Microbes: Are We Ready for What Synthetic Biology Has to Offer?Mol Cell. 2020 May 21;78(4):614-623. doi: 10.1016/j.molcel.2020.03.034. Mol Cell. 2020. PMID: 32442504 Free PMC article. Review.
-
A bumpy road ahead for genetic biocontainment.Nat Commun. 2024 Jan 20;15(1):650. doi: 10.1038/s41467-023-44531-1. Nat Commun. 2024. PMID: 38245521 Free PMC article.
-
Biocontainment of Genetically Engineered Algae.Front Plant Sci. 2022 Mar 2;13:839446. doi: 10.3389/fpls.2022.839446. eCollection 2022. Front Plant Sci. 2022. PMID: 35310623 Free PMC article. Review.
References
-
- Angermayr SA, Gorchs Rovira A, Hellingwerf KJ. Metabolic engineering of cyanobacteria for the synthesis of commodity products. Trends Biotechnol. 2015;33:352–361. - PubMed
-
- Liao JC, Mi L, Pontrelli S, Luo S. Fuelling the future: microbial engineering for the production of sustainable biofuels. Nat Rev Microbiol. 2016;14:288–304. - PubMed
-
- Oliver NJ, Rabinovitch-Deere CA, Carroll AL, Nozzi NE, Case AE, Atsumi S. Cyanobacterial metabolic engineering for biofuel and chemical production. Curr Opin Chem Biol. 2016;35:43–50. - PubMed
-
- Yenkie KM, Wu WZ, Clark RL, Pfleger BF, Root TW, Maravelias CT. A roadmap for the synthesis of separation networks for the recovery of bio-based chemicals: Matching biological and process feasibility. Biotechnol Adv. 2016;34:1362–1383. - PubMed
-
- Molin S, Klemm P, Poulsen LK, Biehl H, Gerdes K, Andersson P. Conditional Suicide System for Containment of Bacteria and Plasmids. Nat Biotechnol. 1987;5:1315–1318.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

