Comparative studies with EPR and MRI on the in vivo tissue redox status estimation using redox-sensitive nitroxyl probes: influence of the choice of the region of interest
- PMID: 29320888
- PMCID: PMC6333207
- DOI: 10.1080/10715762.2018.1427235
Comparative studies with EPR and MRI on the in vivo tissue redox status estimation using redox-sensitive nitroxyl probes: influence of the choice of the region of interest
Abstract
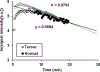
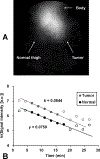
In vivo decay rates of a nitroxyl contrast agent were estimated by a MR redox imaging (MRRI) technique and compared with the decay rates obtained by the electron paramagnetic resonance spectroscopy (EPRS) and imaging (EPRI). MRRI is a dynamic imaging technique employing T1-weighted pulse sequence, which can visualise a nitroxyl-induced enhancement of signal intensity by T1-weighted contrast. EPR techniques can directly measure the paramagnetic nitroxyl radical. Both the squamous cell carcinoma (SCC) tumour-bearing and normal legs of a female C3H mouse were scanned by T1-weighted SPGR sequence at 4.7 T with the nitroxyl radical, carbamoyl-proxyl (CmP), as the contrast agent. Similarly, the time course of CmP in normal muscle and tumour tissues was obtained using a 700-MHz EPR spectrometer with a surface coil. The time course imaging of CmP was also performed by 300 MHz CW EPR imager. EPRS and EPRI gave slower decay rates of CmP compared to the MRRI. Relatively slow decay rate at peripheral region of the tumour tissues, which was found in the image obtained by MRRI, may contribute to the slower decay rates observed by EPRS and/or the EPRI measurements. To reliably determine the tissue redox status from the reduction rates of nitroxyls such as CmP, heterogenic structure in the tumour tissue must be considered. The high spatial and temporal resolution of T1-weighted MRI and the T1-enhancing capabilities of nitroxyls support the use of this method to map tissue redox status which can be a useful biomarker to guide appropriate treatments based on the tumour microenvironment.
Keywords: Electron paramagnetic resonance; magnetic resonance functional imaging; nitroxide radical; redox mapping; redox sensitive contrast agent; tumour physiology.
Figures




Similar articles
-
High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents.Clin Cancer Res. 2006 Apr 15;12(8):2455-62. doi: 10.1158/1078-0432.CCR-05-2747. Clin Cancer Res. 2006. PMID: 16638852
-
Redox imaging of skeletal muscle using in vivo DNP-MRI and its application to an animal model of local inflammation.Free Radic Biol Med. 2015 Dec;89:1097-104. doi: 10.1016/j.freeradbiomed.2015.10.418. Epub 2015 Oct 23. Free Radic Biol Med. 2015. PMID: 26505925
-
3-Carbamoyl-2,2,5,5-tetramethyl-1-pyrrolidinyl-N-oxyl.2008 Apr 30 [updated 2008 Jun 9]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Apr 30 [updated 2008 Jun 9]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641525 Free Books & Documents. Review.
-
15N-Labeled 4-oxo-2,2,6,6-tetramethyl-piperidine-1-oxyl.2008 Apr 30 [updated 2008 Jun 9]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Apr 30 [updated 2008 Jun 9]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641553 Free Books & Documents. Review.
-
3-Carboxy-2,2,5,5-tetramethyl-pyrrolidinyl-N-oxyl.2008 Apr 30 [updated 2008 Jun 9]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Apr 30 [updated 2008 Jun 9]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641530 Free Books & Documents. Review.
Cited by
-
Redox-Sensitive Mapping of a Mouse Tumor Model Using Sparse Projection Sampling of Electron Paramagnetic Resonance.Antioxid Redox Signal. 2022 Jan;36(1-3):57-69. doi: 10.1089/ars.2021.0003. Epub 2021 May 19. Antioxid Redox Signal. 2022. PMID: 33847172 Free PMC article.
-
Effects of oxygen challenging to tissue redox and pO2 status.Free Radic Biol Med. 2019 Jan;130:343-347. doi: 10.1016/j.freeradbiomed.2018.10.454. Epub 2018 Nov 2. Free Radic Biol Med. 2019. PMID: 30391676 Free PMC article.
-
Development of a fast-scan EPR imaging system for highly accelerated free radical imaging.Magn Reson Med. 2019 Aug;82(2):842-853. doi: 10.1002/mrm.27759. Epub 2019 Apr 25. Magn Reson Med. 2019. PMID: 31020713 Free PMC article.
-
Enhancing the Efficacy of Metal-Free MRI Contrast Agents via Conjugating Nitroxides onto PEGylated Cross-Linked Poly(Carboxylate Ester).Adv Sci (Weinh). 2020 Jun 3;7(14):2000467. doi: 10.1002/advs.202000467. eCollection 2020 Jul. Adv Sci (Weinh). 2020. PMID: 32714757 Free PMC article.
-
EPR Everywhere.Appl Magn Reson. 2021;52(8):1113-1139. doi: 10.1007/s00723-020-01304-z. Epub 2021 Jan 24. Appl Magn Reson. 2021. PMID: 33519097 Free PMC article. Review.
References
-
- Brasch RC, Nitecki DE, Brant-Zawadzki M, et al. Brain nuclear magnetic resonance imaging enhanced by a paramagnetic nitroxide contrast agent: preliminary report. Am J Roentgenol. 1983;141:1019–1023. - PubMed
-
- Brasch RC, London DA, Wesbey GE, et al. Work in progress: nuclear magnetic resonance study of a paramagnetic nitroxide contrast agent for enhancement of renal structures in experimental animals. Radiology. 1983;147:773–779. - PubMed
-
- Brasch RC. Work in progress: methods of contrast enhancement for NMR imaging and potential applications. A subject review. Radiology. 1983;147:781–788. - PubMed
-
- Griffeth LK, Rosen GM, Rauckman EJ, et al. Pharmacokinetics of nitroxide NMR contrast agents. Invest Radiol. 1984;19:553–562. - PubMed
-
- Kroll C, Borchert HH. Metabolism of the stable nitroxyl radical 4-oxo-2,2,6, 6-tetramethylpiperidine-N-oxyl (TEMPONE). Eur J Pharm Sci. 1999;8:5–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
