Effects of a culturally specific tobacco cessation intervention among African American Quitline enrollees: a randomized controlled trial
- PMID: 29321008
- PMCID: PMC5763652
- DOI: 10.1186/s12889-017-5015-z
Effects of a culturally specific tobacco cessation intervention among African American Quitline enrollees: a randomized controlled trial
Abstract
Background: African Americans suffer disproportionately from tobacco-related illness and have more difficulty quitting smoking than other racial/ethnic groups. Previous research indicates that African American treatment-seekers are high utilizers of tobacco quitlines, yet cessation rates via quitlines are lower relative to whites. The goal of the present study is to test the effectiveness of adding a culturally specific, video-based, adjunct to standard quitline care. It is hypothesized that the integration of an evidence-based intervention (Pathways to Freedom: Leading the Way to a Smoke-Free Community©; PTF) into quitline services will increase cessation and treatment engagement compared to control conditions, and that effects will be moderated by sociocultural factors (e.g., culturally specific intervention expectancies, acculturation, and ethnic identity).
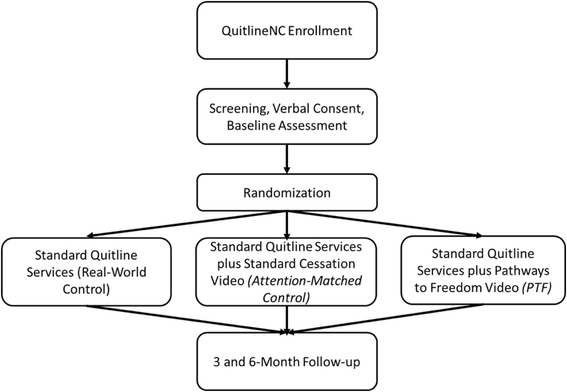
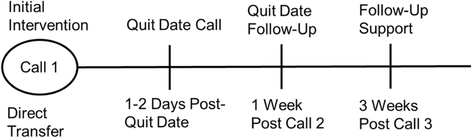
Methods: This study is a 3-arm semi-pragmatic randomized controlled trial (RCT). Participants will be 1050 enrollees in the North Carolina State quitline (QuitlineNC) who self-identify as African American. Usual quitline care includes up to 4 proactive quit coaching calls, website access, and two-weeks of nicotine patch therapy. Eligible study participants will be randomized to receive (1) standard quitline services plus PTF (PTF); (2) quitline services plus a standard tobacco cessation DVD (attention control); or (3) quitline services alone (usual care). Assessments will be conducted at baseline, 3 and 6-months post-enrollment. The primary outcome will be biochemically verified 7 day ppa at 6-months. Generalized linear mixed models (GLMMs) and hierarchical logistic regression will be used to assess the effects of treatment group on cessation outcomes and to test potential moderators.
Discussion: This study will answer questions regarding the implementation and effectiveness of integrating a culturally specific video intervention into a real-world, population-level tobacco intervention. It will also aid our understanding of individual-difference variables that are associated with success. If an incremental benefit is found, this trial will have implications for increasing the responsiveness of tobacco quitlines for African Americans, reducing tobacco cessation disparities, and best practices for improving minority health. In addition, the PTF intervention has the potential for widespread disseminated through quitlines, which are available across the United States.
Trial registration: Clinicaltrials.gov NCT03064971 . Registered on February 22, 2017.
Keywords: African Americans; Cessation; Culturally specific interventions; Disparities; Smoking; Tobacco; Video-based interventions.
Conflict of interest statement
Ethics approval and consent to participate
This study and all consent materials was approved by the Case Western Reserve University Institutional Review Board (IRB) and the Case Comprehensive Cancer Center Protocol Review and Monitoring Committee (PRMC). Participants provide verbal informed consent to participate at the time of quitline enrollment.
Consent for publication
N/A
Competing interests
KC declares that she is employed by Alere Wellbeing and has no other competing interests. The remaining authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Kulak J, Cornelius ME, Fong GT, Giovino GA. Differences in quit attempts and cigarette smoking abstinence between whites and African Americans in the United States: literature review and results from the international tobacco control US survey. Nicotine Tob Res. 2016;18:S79–S87. doi: 10.1093/ntr/ntv228. - DOI - PMC - PubMed
-
- Japuntich SJ, Zelmer ME, Smith SS, Jorenby DE, Valdez JA, Fiore MC, Baker TB, Gustafson DH. Smoking cessation via the internet: a randomized clinical trial of an internet intervention as adjuvant treatment in a smoking cessation intervention. Nicotine Tob Res. 2006;8:S59–S67. doi: 10.1080/14622200601047900. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical