Maternal engineered nanomaterial inhalation during gestation alters the fetal transcriptome
- PMID: 29321036
- PMCID: PMC5763571
- DOI: 10.1186/s12989-017-0239-8
Maternal engineered nanomaterial inhalation during gestation alters the fetal transcriptome
Abstract
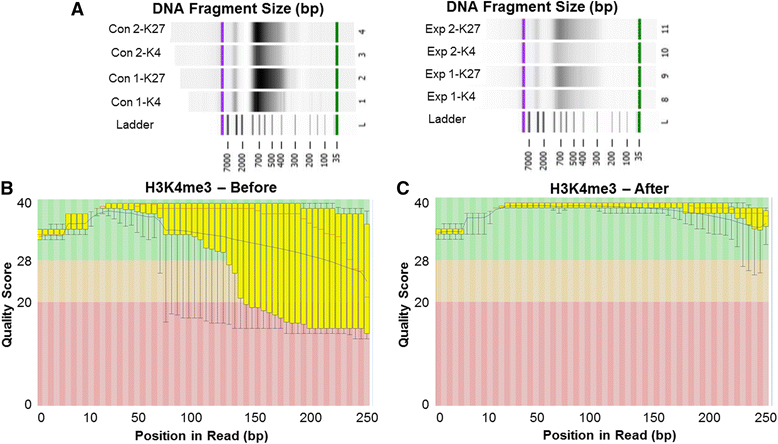
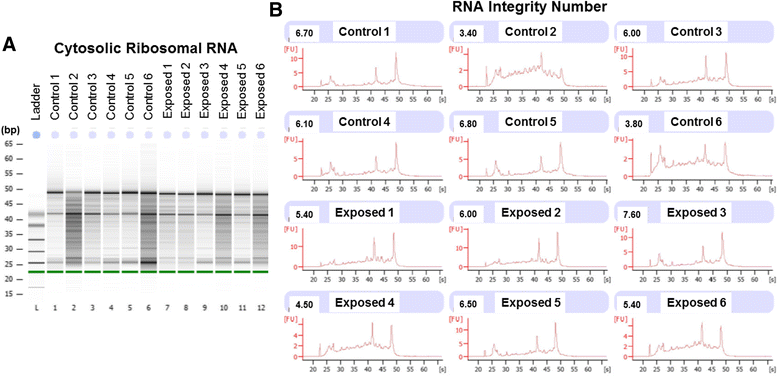
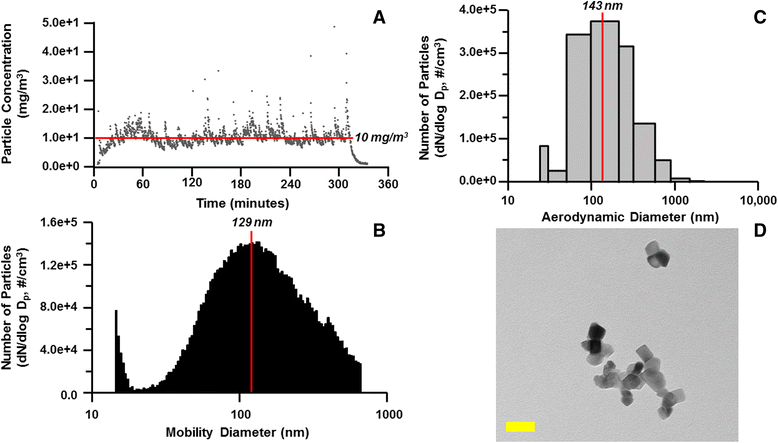
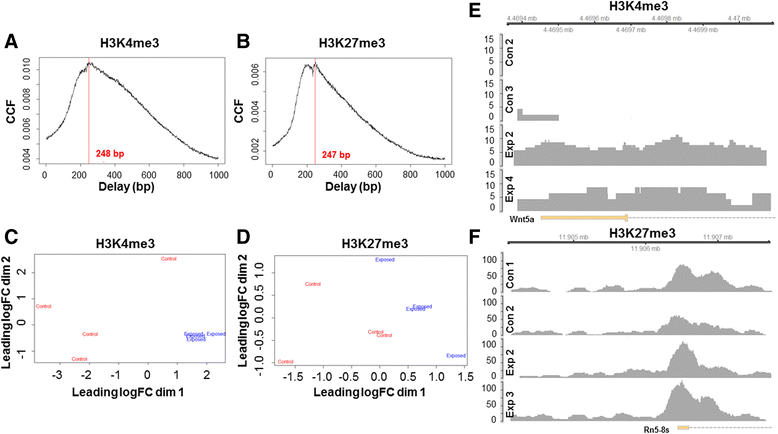
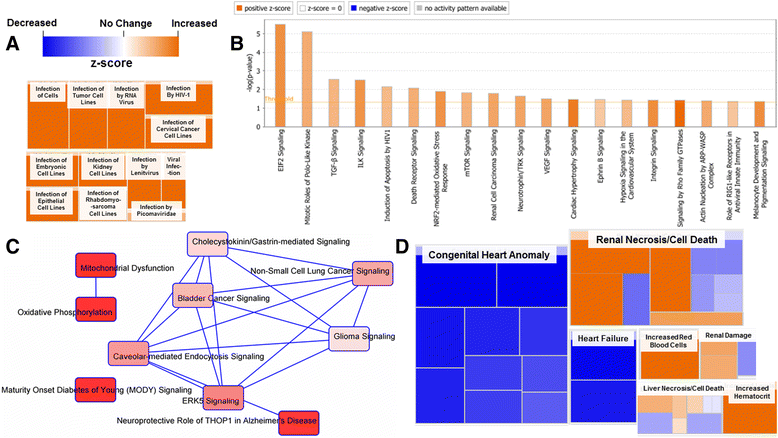
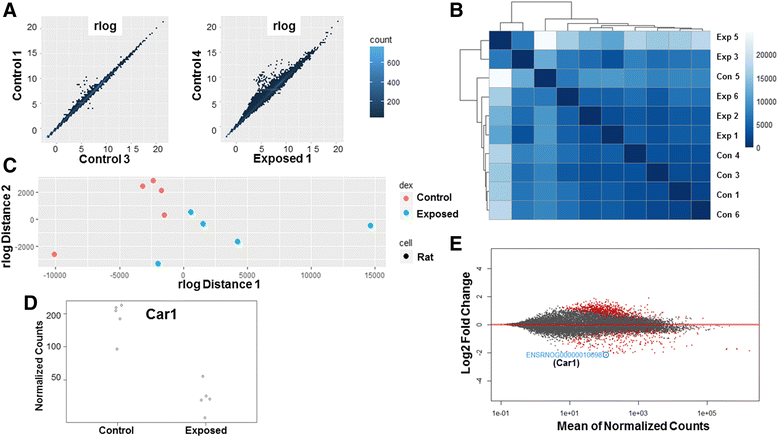
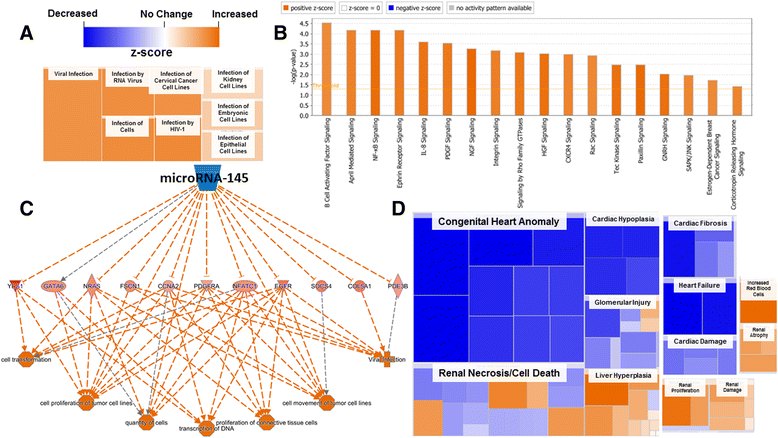
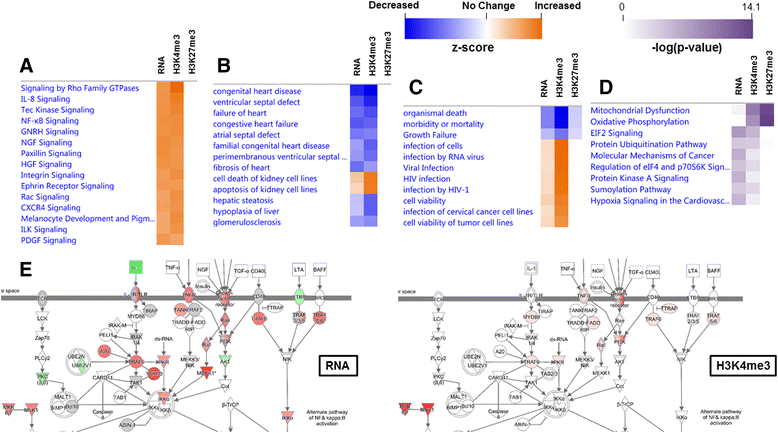
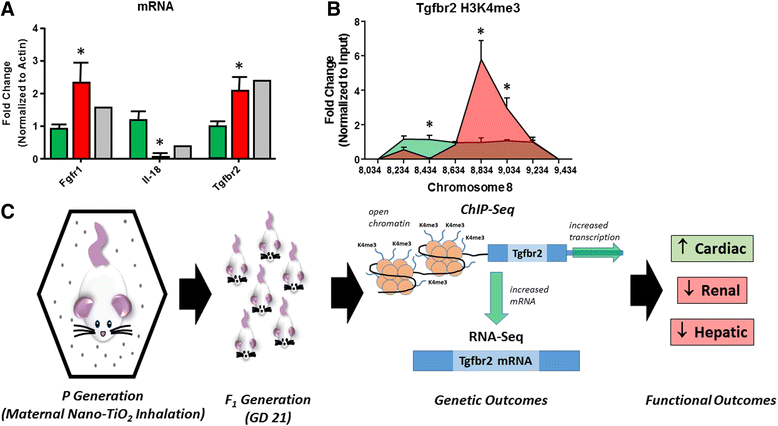
Background: The integration of engineered nanomaterials (ENM) is well-established and widespread in clinical, commercial, and domestic applications. Cardiovascular dysfunctions have been reported in adult populations after exposure to a variety of ENM. As the diversity of these exposures continues to increase, the fetal ramifications of maternal exposures have yet to be determined. We, and others, have explored the consequences of ENM inhalation during gestation and identified many cardiovascular and metabolic outcomes in the F1 generation. The purpose of these studies was to identify genetic alterations in the F1 generation of Sprague-Dawley rats that result from maternal ENM inhalation during gestation. Pregnant dams were exposed to nano-titanium dioxide (nano-TiO2) aerosols (10 ± 0.5 mg/m3) for 7-8 days (calculated, cumulative lung deposition = 217 ± 1 μg) and on GD (gestational day) 20 fetal hearts were isolated. DNA was extracted and immunoprecipitated with modified chromatin marks histone 3 lysine 4 tri-methylation (H3K4me3) and histone 3 lysine 27 tri-methylation (H3K27me3). Following chromatin immunoprecipitation (ChIP), DNA fragments were sequenced. RNA from fetal hearts was purified and prepared for RNA sequencing and transcriptomic analysis. Ingenuity Pathway Analysis (IPA) was then used to identify pathways most modified by gestational ENM exposure.
Results: The results of the sequencing experiments provide initial evidence that significant epigenetic and transcriptomic changes occur in the cardiac tissue of maternal nano-TiO2 exposed progeny. The most notable alterations in major biologic systems included immune adaptation and organismal growth. Changes in normal physiology were linked with other tissues, including liver and kidneys.
Conclusions: These results are the first evidence that maternal ENM inhalation impacts the fetal epigenome.
Keywords: Epigenetics; Fetal; Inhalation; Maternal; Nanomaterial; Nanotechnology; Toxicology.
Conflict of interest statement
Ethics approval and consent to participate
not applicable
Consent for publication
The authors consent to publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
ROS promote epigenetic remodeling and cardiac dysfunction in offspring following maternal engineered nanomaterial (ENM) exposure.Part Fibre Toxicol. 2019 Jun 18;16(1):24. doi: 10.1186/s12989-019-0310-8. Part Fibre Toxicol. 2019. PMID: 31215478 Free PMC article.
-
Maternal engineered nanomaterial exposure and fetal microvascular function: does the Barker hypothesis apply?Am J Obstet Gynecol. 2013 Sep;209(3):227.e1-11. doi: 10.1016/j.ajog.2013.04.036. Epub 2013 Apr 30. Am J Obstet Gynecol. 2013. PMID: 23643573 Free PMC article.
-
Maternal Engineered Nanomaterial Inhalation During Gestation Disrupts Vascular Kisspeptin Reactivity.Toxicol Sci. 2019 Jun 1;169(2):524-533. doi: 10.1093/toxsci/kfz064. Toxicol Sci. 2019. PMID: 30843041 Free PMC article.
-
How can exposure to engineered nanomaterials influence our epigenetic code? A review of the mechanisms and molecular targets.Mutat Res Rev Mutat Res. 2021 Jul-Dec;788:108385. doi: 10.1016/j.mrrev.2021.108385. Epub 2021 Jun 12. Mutat Res Rev Mutat Res. 2021. PMID: 34893164 Review.
-
Environmental and health effects of nanomaterials in nanotextiles and façade coatings.Environ Int. 2011 Aug;37(6):1131-42. doi: 10.1016/j.envint.2011.02.013. Epub 2011 Mar 11. Environ Int. 2011. PMID: 21397331 Review.
Cited by
-
miRNA-378a as a key regulator of cardiovascular health following engineered nanomaterial inhalation exposure.Nanotoxicology. 2019 Jun;13(5):644-663. doi: 10.1080/17435390.2019.1570372. Epub 2019 Feb 1. Nanotoxicology. 2019. PMID: 30704319 Free PMC article.
-
Fetotoxicity of Nanoparticles: Causes and Mechanisms.Nanomaterials (Basel). 2021 Mar 19;11(3):791. doi: 10.3390/nano11030791. Nanomaterials (Basel). 2021. PMID: 33808794 Free PMC article. Review.
-
Nanosafety: An Evolving Concept to Bring the Safest Possible Nanomaterials to Society and Environment.Nanomaterials (Basel). 2022 May 25;12(11):1810. doi: 10.3390/nano12111810. Nanomaterials (Basel). 2022. PMID: 35683670 Free PMC article. Review.
-
Development of coronary dysfunction in adult progeny after maternal engineered nanomaterial inhalation during gestation.Sci Rep. 2021 Sep 29;11(1):19374. doi: 10.1038/s41598-021-98818-8. Sci Rep. 2021. PMID: 34588535 Free PMC article.
-
Should Perturbation of the Preconceptive Environment be Considered a Risk Factor for the Development of Cardiovascular Disease Later in Life?J Am Heart Assoc. 2018 Dec 18;7(24):e011249. doi: 10.1161/JAHA.118.011249. J Am Heart Assoc. 2018. PMID: 30561259 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- DGE-1144676/National Science Foundation/International
- R01 HL128485/HL/NHLBI NIH HHS/United States
- NSF-1003907/National Science Foundation/International
- es024783/ES/NIEHS NIH HHS/United States
- R56 HL128485/HL/NHLBI NIH HHS/United States
- P30 ES005022/ES/NIEHS NIH HHS/United States
- R00 ES024783/ES/NIEHS NIH HHS/United States
- AHA-17PRE33660333/American Heart Association/International
- R01 ES015022/ES/NIEHS NIH HHS/United States
- ES015022/ES/NIEHS NIH HHS/United States
- hl128485/HL/NHLBI NIH HHS/United States
- 13PRE16850066/American Heart Association/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

