Sensorimotor Functional and Structural Networks after Intracerebral Stem Cell Grafts in the Ischemic Mouse Brain
- PMID: 29321138
- PMCID: PMC6705873
- DOI: 10.1523/JNEUROSCI.2715-17.2018
Sensorimotor Functional and Structural Networks after Intracerebral Stem Cell Grafts in the Ischemic Mouse Brain
Abstract
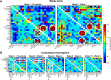
Past investigations on stem cell-mediated recovery after stroke have limited their focus on the extent and morphological development of the ischemic lesion itself over time or on the integration capacity of the stem cell graft ex vivo However, an assessment of the long-term functional and structural improvement in vivo is essential to reliably quantify the regenerative capacity of cell implantation after stroke. We induced ischemic stroke in nude mice and implanted human neural stem cells (H9 derived) into the ipsilateral cortex in the acute phase. Functional and structural connectivity changes of the sensorimotor network were noninvasively monitored using magnetic resonance imaging for 3 months after stem cell implantation. A sharp decrease of the functional sensorimotor network extended even to the contralateral hemisphere, persisting for the whole 12 weeks of observation. In mice with stem cell implantation, functional networks were stabilized early on, pointing to a paracrine effect as an early supportive mechanism of the graft. This stabilization required the persistent vitality of the stem cells, monitored by bioluminescence imaging. Thus, we also observed deterioration of the early network stabilization upon vitality loss of the graft after a few weeks. Structural connectivity analysis showed fiber-density increases between the cortex and white matter regions occurring predominantly on the ischemic hemisphere. These fiber-density changes were nearly the same for both study groups. This motivated us to hypothesize that the stem cells can influence, via early paracrine effect, the functional networks, while observed structural changes are mainly stimulated by the ischemic event.SIGNIFICANCE STATEMENT In recent years, research on strokes has made a shift away from a focus on immediate ischemic effects and towards an emphasis on the long-range effects of the lesion on the whole brain. Outcome improvements in stem cell therapies also require the understanding of their influence on the whole-brain networks. Here, we have longitudinally and noninvasively monitored the structural and functional network alterations in the mouse model of focal cerebral ischemia. Structural changes of fiber-density increases are stimulated in the endogenous tissue without further modulation by the stem cells, while functional networks are stabilized by the stem cells via a paracrine effect. These results will help decipher the underlying networks of brain plasticity in response to cerebral lesions and offer clues to unravelling the mystery of how stem cells mediate regeneration.
Keywords: functional networks; mouse; stem cell implantation; stroke; structural networks.
Copyright © 2018 the authors 0270-6474/18/381648-14$15.00/0.
Figures







References
-
- Adamczak J, Aswendt M, Kreutzer C, Rotheneichner P, Riou A, Selt M, Beyrau A, Uhlenküken U, Diedenhofen M, Nelles M, Aigner L, Couillard-Despres S, Hoehn M (2017) Neurogenesis upregulation on the healthy hemisphere after stroke enhances compensation for age-dependent decrease of basal neurogenesis. Neurobiol Dis 99:47–57. 10.1016/j.nbd.2016.12.015 - DOI - PubMed
-
- Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Jametti LP, Kilic E, Kilic U, Salani G, Brambilla E, West MJ, Comi G, Martino G, Hermann DM (2009) Delayed postischemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain 132:2239–2251. 10.1093/brain/awp174 - DOI - PubMed
-
- Bacigaluppi M, Russo GL, Peruzzotti-Jametti L, Rossi S, Sandrone S, Butti E, De Ceglia R, Bergamaschi A, Motta C, Gallizioli M, Studer V, Colombo E, Farina C, Comi G, Politi LS, Muzio L, Villani C, Invernizzi RW, Hermann DM, Centonze D, et al. (2016) Neural stem cell transplantation induces stroke recovery by upregulating glutamate transporter GLT-1 in astrocytes. J Neurosci 36:10529–10544. 10.1523/JNEUROSCI.1643-16.2016 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
