Efficacy of intensive multidisciplinary rehabilitation in Parkinson's disease: a randomised controlled study
- PMID: 29321141
- PMCID: PMC6204945
- DOI: 10.1136/jnnp-2017-316437
Efficacy of intensive multidisciplinary rehabilitation in Parkinson's disease: a randomised controlled study
Abstract
Objective: To evaluate whether a 4-week multidisciplinary, aerobic, motor-cognitive and intensive rehabilitation treatment (MIRT) improves the quality of life (QoL) of patients with Parkinson's disease (PD), in the short-term and long-term period.
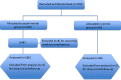
Methods: This is a prospective, parallel-group, single-centre, single-blind, randomised clinical trial (ClinicalTrials.gov NCT02756676). 186 patients with PD, assigned to experimental group, underwent MIRT; conversely, 48 patients, assigned to control group, did not receive rehabilitation. Parkinson's Disease Questionnaire-39 was assessed 2 (T0), 10 (T1) and 18 (T2, only experimental group) weeks after the enrolment. We compared T1 versus T0 scores within subjects and delta scores (T1-T0) between subjects. To investigate the long-term effects, we compared T2 and T0 scores in the experimental group.
Results: At T0, no between-group differences in the Global Index Score (GBI) were observed (experimental group: 43.6±21.4, controls: 41.6±22.9, P=0.50). At T1, we did not find significant changes in controls (delta score: 1.2±9.9, P=0.23), and we found an improvement in GBI in the experimental group (delta score: -8.3±18.0, P<0.0001), significant also between subjects (P<0.0001). Comparing T2 versus T0 in the experimental group, the GBI maintained a significant improvement (delta score: -4.8±17.5, P<0.0001).
Conclusions: A rehabilitation treatment such as MIRT could improve QoL in patients with PD in the short-term and long-term period. Even though the single-blind design and the possible role of the placebo effect on the conclusive results must be considered as limitations of this study, the improvement in outcome measure, also maintained after a 3-month follow-up period, suggests the effectiveness of MIRT on the QoL.
Clinical trial registration: NCT02756676: Pre-results.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment in
-
Intensive inpatient rehabilitation for persons with Parkinson's disease: last resort or pre-emptive strike?J Neurol Neurosurg Psychiatry. 2018 Aug;89(8):795-796. doi: 10.1136/jnnp-2017-317812. Epub 2018 Mar 13. J Neurol Neurosurg Psychiatry. 2018. PMID: 29535145 No abstract available.
References
-
- Chaudhuri KR, Healy DG, Schapira AH. National Institute for Clinical Excellence. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 2006;5:235–45. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical