Chronic skin inflammation accelerates macrophage cholesterol crystal formation and atherosclerosis
- PMID: 29321372
- PMCID: PMC5821196
- DOI: 10.1172/jci.insight.97179
Chronic skin inflammation accelerates macrophage cholesterol crystal formation and atherosclerosis
Abstract
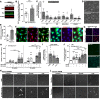
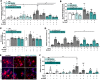
Inflammation is critical to atherogenesis. Psoriasis is a chronic inflammatory skin disease that accelerates atherosclerosis in humans and provides a compelling model to understand potential pathways linking these diseases. A murine model capturing the vascular and metabolic diseases in psoriasis would accelerate our understanding and provide a platform to test emerging therapies. We aimed to characterize a new murine model of skin inflammation (Rac1V12) from a cardiovascular standpoint to identify novel atherosclerotic signaling pathways modulated in chronic skin inflammation. The RacV12 psoriasis mouse resembled the human disease state, including presence of systemic inflammation, dyslipidemia, and cardiometabolic dysfunction. Psoriasis macrophages had a proatherosclerotic phenotype with increased lipid uptake and foam cell formation, and also showed a 6-fold increase in cholesterol crystal formation. We generated a triple-genetic K14-RacV12-/+/Srb1-/-/ApoER61H/H mouse and confirmed psoriasis accelerates atherogenesis (~7-fold increase). Finally, we noted a 60% reduction in superoxide dismutase 2 (SOD2) expression in human psoriasis macrophages. When SOD2 activity was restored in macrophages, their proatherogenic phenotype reversed. We demonstrate that the K14-RacV12 murine model captures the cardiometabolic dysfunction and accelerates vascular disease observed in chronic inflammation and that skin inflammation induces a proatherosclerotic macrophage phenotype with impaired SOD2 function, which associated with accelerated atherogenesis.
Keywords: Atherosclerosis; Cardiology; Cardiovascular disease; Inflammation; Macrophages.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

