Development of an airway mucus defect in the cystic fibrosis rat
- PMID: 29321377
- PMCID: PMC5821204
- DOI: 10.1172/jci.insight.97199
Development of an airway mucus defect in the cystic fibrosis rat
Abstract
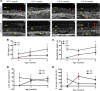
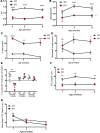
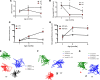
The mechanisms underlying the development and natural progression of the airway mucus defect in cystic fibrosis (CF) remain largely unclear. New animal models of CF, coupled with imaging using micro-optical coherence tomography, can lead to insights regarding these questions. The Cftr-/- (KO) rat allows for longitudinal examination of the development and progression of airway mucus abnormalities. The KO rat exhibits decreased periciliary depth, hyperacidic pH, and increased mucus solid content percentage; however, the transport rates and viscoelastic properties of the mucus are unaffected until the KO rat ages. Airway submucosal gland hypertrophy develops in the KO rat by 6 months of age. Only then does it induce increased mucus viscosity, collapse of the periciliary layer, and delayed mucociliary transport; stimulation of gland secretion potentiates this evolution. These findings could be reversed by bicarbonate repletion but not pH correction without counterion donation. These studies demonstrate that abnormal surface epithelium in CF does not cause delayed mucus transport in the absence of functional gland secretions. Furthermore, abnormal bicarbonate transport represents a specific target for restoring mucus clearance, independent of effects on periciliary collapse. Thus, mature airway secretions are required to manifest the CF defect primed by airway dehydration and bicarbonate deficiency.
Keywords: Cell Biology; Epithelial transport of ions and water; Pulmonology.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

