Xeno-free pre-vascularized spheroids for therapeutic applications
- PMID: 29321569
- PMCID: PMC5762877
- DOI: 10.1038/s41598-017-18431-6
Xeno-free pre-vascularized spheroids for therapeutic applications
Abstract
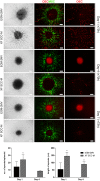
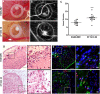
Spheroid culture has gained increasing popularity, arising as a promising tool for regenerative medicine applications. Importantly, spheroids may present advantages over single-cell suspensions in cell-based therapies (CT). Unfortunately, most growth media used for spheroid culture contain animal origin-components, such as fetal bovine serum (FBS). The presence of FBS compromises the safety of CT and presents economic and ethical constraints. SCC (supplement for cell culture) is a novel xeno-free (XF) industrial cell culture supplement, derived from well-controlled pooled human plasma and processed under good manufacturing practice rules. Here, we developed a XF SCC-based formulation for 2D-culture of outgrowth endothelial cells (OEC), and then used it for generating co-culture spheroids of OEC and mesenchymal stem cells (MSC). XF MSC-OEC spheroids were characterized in detail and compared to spheroids cultured in FBS-supplemented medium. XF spheroids presented comparable integrity, size and morphology as the reference culture. The use of both media resulted in spheroids with similar structure, abundant extracellular matrix deposition and specific patterns of OEC distribution and organization. Notably, XF spheroids presented significantly enhanced angiogenic potential, both in vitro (fibrin sprouting assay) and in vivo (CAM assay). These findings are particularly promising in the context of potential therapeutic applications.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

