Asymmetric reproductive interference: The consequences of cross-pollination on reproductive success in sexual-apomictic populations of Potentilla puberula (Rosaceae)
- PMID: 29321878
- PMCID: PMC5756837
- DOI: 10.1002/ece3.3684
Asymmetric reproductive interference: The consequences of cross-pollination on reproductive success in sexual-apomictic populations of Potentilla puberula (Rosaceae)
Abstract
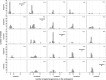
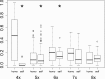
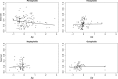
Apomixis evolves from a sexual background and usually is linked to polyploidization. Pseudogamous gametophytic apomicts, which require a fertilization to initiate seed development, of various ploidy levels frequently co-occur with their lower-ploid sexual ancestors, but the stability of such mixed populations is affected by reproductive interferences mediated by cross-pollination. Thereby, reproductive success of crosses depends on the difference in ploidy levels of mating partners, that is, on tolerance of deviation from the balanced ratio of maternal versus paternal genomes. Quality of pollen can further affect reproductive success in intercytotype pollinations. Cross-fertilization, however, can be avoided by selfing which may be induced upon pollination with mixtures of self- and cross-pollen (i.e., mentor effects). We tested for reproductive compatibility of naturally co-occurring tetraploid sexuals and penta- to octoploid apomicts in the rosaceous species Potentilla puberula by means of controlled crosses. We estimated the role of selfing as a crossing barrier and effects of self- and cross-pollen quality as well as maternal: paternal genomic ratios in the endosperm on reproductive success. Cross-fertilization of sexuals by apomicts was not blocked by selfing, and seed set was reduced in hetero- compared to homoploid crosses. Thereby, seed set was negatively related to deviations from balanced parental genomic ratios in the endosperm. In contrast, seed set in the apomictic cytotypes was not reduced in hetero- compared to homoploid crosses. Thus, apomictic cytotypes either avoided intercytotype cross-fertilization through selfing, tolerated intercytotype cross-fertilizations without negative effects on reproductive success, or even benefitted from higher pollen quality in intercytotype pollinations. Our experiment provides evidence for asymmetric reproductive interference, in favor of the apomicts, with significantly reduced seed set of sexuals in cytologically mixed populations, whereas seed set in apomicts was not affected. Incompleteness of crossing barriers further indicated at least partial losses of a parental genomic endosperm balance requirement.
Keywords: apomixis; crossing barrier; genomic endosperm balance; pollen; polyploidy; selfing.
Figures




References
-
- Asker, S. (1970). Apomictic biotypes in Potentilla intermedia and P. norvegica . Hereditas, 66, 101–108. https://doi.org/10.1111/j.1601-5223.1970.tb02337.x - DOI
-
- Asker, S. (1980). Gametophytic apomixis: Elements and genetic regulation. Hereditas, 93, 277–293. https://doi.org/10.1111/j.1601-5223.1980.tb01367.x - DOI
-
- Asker, S. E. , & Jerling, L. (1992). Apomixis in plants (p. 298). Boca Raton, FL: CRC Press.
-
- Baack, E. J. (2004). Cytyotype segregation on regional and microgeographic scales in snow buttercups (Ranunculus adoneus: Ranunculaceae). American Journal of Botany, 91, 1783–1788. https://doi.org/10.3732/ajb.91.11.1783 - DOI - PubMed
-
- Barcaccia, G. , Arzenton, F. , Sharbel, T. F. , Varotto, S. , Parrini, P. , & Lucchin, M. (2006). Genetic diversity and reproductive biology in ecotypes of the facultative apomict Hypericum perforatum L. Heredity, 96, 322–334. https://doi.org/10.1038/sj.hdy.6800808 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

