Inhibition of aqueous extracts of Solanum nigrum (AESN) on oral cancer through regulation of mitochondrial fission
- PMID: 29322012
- PMCID: PMC5756017
- DOI: 10.1016/j.jtcme.2017.05.011
Inhibition of aqueous extracts of Solanum nigrum (AESN) on oral cancer through regulation of mitochondrial fission
Abstract
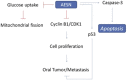
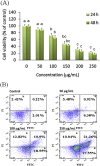
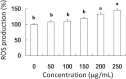
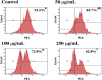
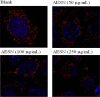
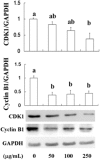
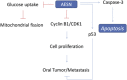
The present study is designed to investigate the anti-oral cancer properties of Solanum nigrum on oral squamous cell carcinoma. S. nigrum is a Chinese herb used for suppression of various cancers. However, the inhibition of S. nigrum on oral cancer is unclear. Therefore, human oral squamous cancer cells (SCC)-4 were used to evaluate the effect of aqueous extracts of S. nigrum (AESN) on cancer cell proliferation, cell cycle, mitochondrial function and apoptosis. The SCC-4 cells were treated by AESN to evaluate the inhibition of cell proliferation and mitochondrial function in vitro. Our results suggested that AESN markedly increased reactive oxygen species production. AESN also promoted caspase-9 and caspase-3 activation and subsequent triggering of the mitochondrial apoptotic pathway. The inhibition of glucose uptake was alleviated mediated by a dose-dependent manner in SCC-4 cells with AESN treatment for 24 h, resulting in mitochondrial fission. These results suggested that AESN has potential to be used as a functional food in adjuvant chemotherapy for treating human oral cancer by suppression of mitochondrial function.
Keywords: Apoptosis; Mitochondrial fission; Mitochondrial function; Oral squamous cell carcinoma; Solanum nigrum.
Figures
Similar articles
-
Anti-Cancer Activity of Solanum nigrum (AESN) through Suppression of Mitochondrial Function and Epithelial-Mesenchymal Transition (EMT) in Breast Cancer Cells.Molecules. 2016 Apr 28;21(5):553. doi: 10.3390/molecules21050553. Molecules. 2016. PMID: 27136519 Free PMC article.
-
Solanum nigrum Protects against Hepatic Fibrosis via Suppression of Hyperglycemia in High-Fat/Ethanol Diet-Induced Rats.Molecules. 2016 Feb 25;21(3):269. doi: 10.3390/molecules21030269. Molecules. 2016. PMID: 26927042 Free PMC article.
-
Protective effects of aqueous extract of Solanum nigrum Linn. leaves in rat models of oral mucositis.ScientificWorldJournal. 2014;2014:345939. doi: 10.1155/2014/345939. Epub 2014 Nov 20. ScientificWorldJournal. 2014. PMID: 25506066 Free PMC article.
-
Current development in integrative therapy of traditional Chinese medicine for cancer treatment: A mini-review.J Tradit Complement Med. 2019 Jul 9;10(5):429-433. doi: 10.1016/j.jtcme.2019.07.001. eCollection 2020 Sep. J Tradit Complement Med. 2019. PMID: 32953557 Free PMC article. Review.
-
Solanum nigrum Linn.: Advances in anti-cancer activity and mechanism in digestive system tumors.Med Oncol. 2023 Sep 29;40(11):311. doi: 10.1007/s12032-023-02167-7. Med Oncol. 2023. PMID: 37775552 Review.
Cited by
-
Hidden in Plants-A Review of the Anticancer Potential of the Solanaceae Family in In Vitro and In Vivo Studies.Cancers (Basel). 2022 Mar 11;14(6):1455. doi: 10.3390/cancers14061455. Cancers (Basel). 2022. PMID: 35326606 Free PMC article. Review.
-
Haimufang decoction, a Chinese medicine formula for lung cancer, arrests cell cycle, stimulates apoptosis in NCI-H1975 cells, and induces M1 polarization in RAW 264.7 macrophage cells.BMC Complement Med Ther. 2020 Aug 5;20(1):243. doi: 10.1186/s12906-020-03031-1. BMC Complement Med Ther. 2020. PMID: 32758223 Free PMC article.
-
Probing the Antitumor Mechanism of Solanum nigrum L. Aqueous Extract against Human Breast Cancer MCF7 Cells.Bioengineering (Basel). 2019 Dec 11;6(4):112. doi: 10.3390/bioengineering6040112. Bioengineering (Basel). 2019. PMID: 31835887 Free PMC article.
-
Main chemical constituents and mechanism of anti-tumor action of Solanum nigrum L.Cancer Med. 2024 Aug;13(16):e7314. doi: 10.1002/cam4.7314. Cancer Med. 2024. PMID: 39155844 Free PMC article. Review.
-
Exploring the components and mechanism of Solanum nigrum L. for colon cancer treatment based on network pharmacology and molecular docking.Front Oncol. 2023 Mar 8;13:1111799. doi: 10.3389/fonc.2023.1111799. eCollection 2023. Front Oncol. 2023. PMID: 36969029 Free PMC article.
References
-
- Parkin D.M., Bray F., Ferlay J., Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. - PubMed
-
- Blot W.J., McLaughlin J.K., Winn D.M. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287. - PubMed
-
- McLaughlin J.K., Gridley G., Block G. Dietary factors in oral and pharyngeal cancer. J Nat Cancer Ins. 1988;80:1237–1243. - PubMed
-
- Pintos J., Franco E.L., Kowalski L.P., Oliveira B.V., Curado M.P. Use of wood stoves and risk of cancers of the upper aero-digestive tract: a case-control study. Int J Epidemiol. 1998;27:936–940. - PubMed
-
- Klaunig J.E., Kamendulis L.M., Hocevar B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38:96–109. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
