Cold-inducible RNA binding protein in cancer and inflammation
- PMID: 29322631
- PMCID: PMC5886743
- DOI: 10.1002/wrna.1462
Cold-inducible RNA binding protein in cancer and inflammation
Abstract
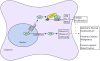
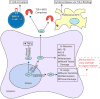
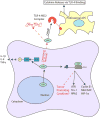
RNA binding proteins (RBPs) play key roles in RNA dynamics, including subcellular localization, translational efficiency and metabolism. Cold-inducible RNA binding protein (CIRP) is a stress-induced protein that was initially described as a DNA damage-induced transcript (A18 hnRNP), as well as a cold-shock domain containing cold-stress response protein (CIRBP) that alters the translational efficiency of its target messenger RNAs (mRNAs). This review summarizes recent work on the roles of CIRP in the context of inflammation and cancer. The function of CIRP in cancer appeared to be solely driven though its functions as an RBP that targeted cancer-associated mRNAs, but it is increasingly clear that CIRP also modulates inflammation. Several recent studies highlight roles for CIRP in immune responses, ranging from sepsis to wound healing and tumor-promoting inflammation. While modulating inflammation is an established role for RBPs that target cytokine mRNAs, CIRP appears to modulate inflammation by several different mechanisms. CIRP has been found in serum, where it binds the TLR4-MD2 complex, acting as a Damage-associated molecular pattern (DAMP). CIRP activates the NF-κB pathway, increasing phosphorylation of Iκκ and IκBα, and stabilizes mRNAs encoding pro-inflammatory cytokines. While CIRP promotes higher levels of pro-inflammatory cytokines in certain cancers, it also decreases inflammation to accelerate wound healing. This dichotomy suggests that the influence of CIRP on inflammation is context dependent and highlights the importance of detailing the mechanisms by which CIRP modulates inflammation. WIREs RNA 2018, 9:e1462. doi: 10.1002/wrna.1462 This article is categorized under: RNA in Disease and Development > RNA in Disease RNA Interactions with Proteins and Other Molecules > Protein-RNA Interactions: Functional Implications.
Keywords: A18 hnRNP; CIRP; RNA binding protein; cancer; inflammation; stress.
© 2018 Wiley Periodicals, Inc.
Figures

References
-
- Artero-Castro A, Callejas FB, Castellvi J, Kondoh H, Carnero A, Fernández-Marcos PJ, et al. Lleonart ME. Cold-inducible RNA-binding protein bypasses replicative senescence in primary cells through extracellular signal-regulated kinase 1 and 2 activation. Molecular and cellular biology. 2009;29(7):1855–1868. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

