Functional Antibody Response Against V1V2 and V3 of HIV gp120 in the VAX003 and VAX004 Vaccine Trials
- PMID: 29323175
- PMCID: PMC5765017
- DOI: 10.1038/s41598-017-18863-0
Functional Antibody Response Against V1V2 and V3 of HIV gp120 in the VAX003 and VAX004 Vaccine Trials
Abstract
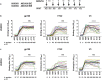
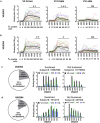
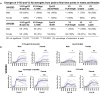
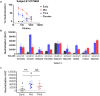
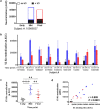
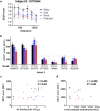
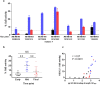
Immunization with HIV AIDSVAX gp120 vaccines in the phase III VAX003 and VAX004 trials did not confer protection. To understand the shortcomings in antibody (Ab) responses induced by these vaccines, we evaluated the kinetics of Ab responses to the V1V2 and V3 regions of gp120 and the induction of Ab-mediated antiviral functions during the course of 7 vaccinations over a 30.5-month period. Plasma samples from VAX003 and VAX004 vaccinees and placebo recipients were measured for ELISA-binding Abs and for virus neutralization, Ab-dependent cellular phagocytosis (ADCP), and Ab-dependent cellular cytotoxicity (ADCC). Ab responses to V1V2 and V3 peaked after 3 to 4 immunizations and declined after 5 to 7 immunizations. The deteriorating responses were most evident against epitopes in the underside of the V1V2 β-barrel and in the V3 crown. Correspondingly, vaccinees demonstrated higher neutralization against SF162 pseudovirus sensitive to anti-V1V2 and anti-V3 Abs after 3 or 4 immunizations than after 7 immunizations. Higher levels of ADCP and ADCC were also observed at early or mid-time points as compared with the final time point. Hence, VAX003 and VAX004 vaccinees generated V1V2- and V3-binding Abs and functional Abs after 3 to 4 immunizations, but subsequent boosts did not maintain these responses.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

