Structure and dynamics of GPCR signaling complexes
- PMID: 29323277
- PMCID: PMC6535338
- DOI: 10.1038/s41594-017-0011-7
Structure and dynamics of GPCR signaling complexes
Abstract
G-protein-coupled receptors (GPCRs) relay numerous extracellular signals by triggering intracellular signaling through coupling with G proteins and arrestins. Recent breakthroughs in the structural determination of GPCRs and GPCR-transducer complexes represent important steps toward deciphering GPCR signal transduction at a molecular level. A full understanding of the molecular basis of GPCR-mediated signaling requires elucidation of the dynamics of receptors and their transducer complexes as well as their energy landscapes and conformational transition rates. Here, we summarize current insights into the structural plasticity of GPCR-G-protein and GPCR-arrestin complexes that underlies the regulation of the receptor's intracellular signaling profile.
Conflict of interest statement
Competing interests
B.K.K. is a cofounder and consultant for ConfometRx, Inc.
Figures
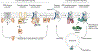
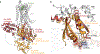
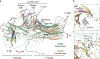
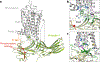
References
-
- Wu F, Song G, de Graaf C & Stevens RC Structure and function of peptide-binding G protein-coupled receptors. J. Mol. Biol 429, 2726–2745 (2017). - PubMed
-
- Shonberg J, Kling RC, Gmeiner P & Löber S GPCR crystal structures: medicinal chemistry in the pocket. Bioorg. Med. Chem 23, 3880–3906 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

