Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells
- PMID: 29323295
- PMCID: PMC6047743
- DOI: 10.1038/nature25154
Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells
Abstract
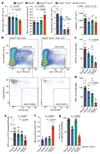
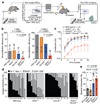
Haematopoietic stem cells renew blood. Accumulation of DNA damage in these cells promotes their decline, while misrepair of this damage initiates malignancies. Here we describe the features and mutational landscape of DNA damage caused by acetaldehyde, an endogenous and alcohol-derived metabolite. This damage results in DNA double-stranded breaks that, despite stimulating recombination repair, also cause chromosome rearrangements. We combined transplantation of single haematopoietic stem cells with whole-genome sequencing to show that this damage occurs in stem cells, leading to deletions and rearrangements that are indicative of microhomology-mediated end-joining repair. Moreover, deletion of p53 completely rescues the survival of aldehyde-stressed and mutated haematopoietic stem cells, but does not change the pattern or the intensity of genome instability within individual stem cells. These findings characterize the mutation of the stem-cell genome by an alcohol-derived and endogenous source of DNA damage. Furthermore, we identify how the choice of DNA-repair pathway and a stringent p53 response limit the transmission of aldehyde-induced mutations in stem cells.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


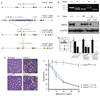


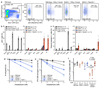



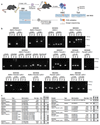
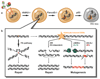




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

