MukB ATPases are regulated independently by the N- and C-terminal domains of MukF kleisin
- PMID: 29323635
- PMCID: PMC5812716
- DOI: 10.7554/eLife.31522
MukB ATPases are regulated independently by the N- and C-terminal domains of MukF kleisin
Abstract
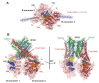
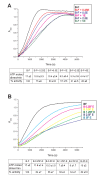
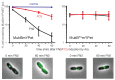
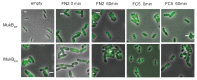
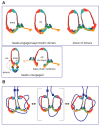
The Escherichia coli SMC complex, MukBEF, acts in chromosome segregation. MukBEF shares the distinctive architecture of other SMC complexes, with one prominent difference; unlike other kleisins, MukF forms dimers through its N-terminal domain. We show that a 4-helix bundle adjacent to the MukF dimerisation domain interacts functionally with the MukB coiled-coiled 'neck' adjacent to the ATPase head. We propose that this interaction leads to an asymmetric tripartite complex, as in other SMC complexes. Since MukF dimerisation is preserved during this interaction, MukF directs the formation of dimer of dimer MukBEF complexes, observed previously in vivo. The MukF N- and C-terminal domains stimulate MukB ATPase independently and additively. We demonstrate that impairment of the MukF interaction with MukB in vivo leads to ATP hydrolysis-dependent release of MukBEF complexes from chromosomes.
Keywords: E. coli; MukBEF; SMC proteins; chromosomes; genes; molecular machines.
© 2018, Zawadzka et al.
Conflict of interest statement
KZ, PZ, RB, KR, FW, LA No competing interests declared, DS Reviewing editor, <italic>eLife</italic>
Figures











References
-
- Beckouët F, Srinivasan M, Roig MB, Chan KL, Scheinost JC, Batty P, Hu B, Petela N, Gligoris T, Smith AC, Strmecki L, Rowland BD, Nasmyth K. Releasing activity disengages cohesin's Smc3/Scc1 interface in a process blocked by acetylation. Molecular Cell. 2016;61:563–574. doi: 10.1016/j.molcel.2016.01.026. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

