FREQUENT SUBCLINICAL MACULAR CHANGES IN COMBINED BRAF/MEK INHIBITION WITH HIGH-DOSE HYDROXYCHLOROQUINE AS TREATMENT FOR ADVANCED METASTATIC BRAF MUTANT MELANOMA: Preliminary Results From a Phase I/II Clinical Treatment Trial
- PMID: 29324592
- PMCID: PMC6039280
- DOI: 10.1097/IAE.0000000000002027
FREQUENT SUBCLINICAL MACULAR CHANGES IN COMBINED BRAF/MEK INHIBITION WITH HIGH-DOSE HYDROXYCHLOROQUINE AS TREATMENT FOR ADVANCED METASTATIC BRAF MUTANT MELANOMA: Preliminary Results From a Phase I/II Clinical Treatment Trial
Abstract
Purpose: To assess the potential ocular toxicity of a combined BRAF inhibition (BRAFi) + MEK inhibition (MEKi) + hydroxychloroquine (HCQ) regime used to treat metastatic BRAF mutant melanoma.
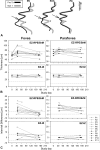
Methods: Patients with stage IV metastatic melanoma and BRAF V600E mutations (n = 11, 31-68 years of age) were included. Treatment was with oral dabrafenib, 150 mg bid, trametinib, 2 mg/day, and HCQ, 400 mg to 600 mg bid. An ophthalmic examination, spectral domain optical coherence tomography, near-infrared and short-wavelength fundus autofluorescence, and static perimetry were performed at baseline, 1 month, and q/6 months after treatment.
Results: There were no clinically significant ocular events; there was no ocular inflammation. The only medication-related change was a separation of the photoreceptor outer segment tip from the apical retinal pigment epithelium that could be traced from the fovea to the perifoveal retina noted in 9/11 (82%) of the patients. There were no changes in retinal pigment epithelium melanization or lipofuscin content by near-infrared fundus autofluorescence and short-wavelength fundus autofluorescence, respectively. There were no inner retinal or outer nuclear layer changes. Visual acuities and sensitivities were unchanged.
Conclusion: BRAFi (trametinib) + MEKi (dabrafenib) + HCQ causes very frequent, subclinical separation of the photoreceptor outer segment from the apical retinal pigment epithelium without inner retinal changes or signs of inflammation. The changes suggest interference with the maintenance of the outer retinal barrier and/or phagocytic/pump functions of the retinal pigment epithelium by effective MEK inhibition.
Conflict of interest statement
None of the authors has any conflicting interests to disclose.
Figures



References
-
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–114. - PubMed
-
- Pasquali S, Chiarion-Sileni V, Rossi CR, Mocellin S. Immune checkpoint inhibitors and targeted therapies for metastatic melanoma: a network meta-analysis. Cancer Treat Rev. 2017;54:34–42. - PubMed
-
- Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. Lancet. 2014;383:816–827. - PubMed
-
- Ugurel S, Röhmel J, Ascierto PA, et al. Survival of patients with advanced metastatic melanoma: the impact of novel therapies. Eur J Cancer. 2016;53:125–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

