Listeria monocytogenes: The Impact of Cell Death on Infection and Immunity
- PMID: 29324702
- PMCID: PMC5874734
- DOI: 10.3390/pathogens7010008
Listeria monocytogenes: The Impact of Cell Death on Infection and Immunity
Abstract
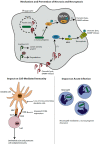
Listeria monocytogenes has evolved exquisite mechanisms for invading host cells and spreading from cell-to-cell to ensure maintenance of its intracellular lifecycle. As such, it is not surprising that loss of the intracellular replication niche through induction of host cell death has significant implications on the development of disease and the subsequent immune response. Although L. monocytogenes can activate multiple pathways of host cell death, including necrosis, apoptosis, and pyroptosis, like most intracellular pathogens L. monocytogenes has evolved a series of adaptations that minimize host cell death to promote its virulence. Understanding how L. monocytogenes modulates cell death during infection could lead to novel therapeutic approaches. In addition, as L. monocytogenes is currently being developed as a tumor immunotherapy platform, understanding how cell death pathways influence the priming and quality of cell-mediated immunity is critical. This review will focus on the mechanisms by which L. monocytogenes modulates cell death, as well as the implications of cell death on acute infection and the generation of adaptive immunity.
Keywords: CD8+ T-cells; Listeria monocytogenes; apoptosis; cell death; cell-mediated immunity; necrosis; pyroptosis.
Conflict of interest statement
The authors declare no conflict of interests.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

