Evaluation of a New Survivin ELISA and UBC® Rapid for the Detection of Bladder Cancer in Urine
- PMID: 29324722
- PMCID: PMC5796175
- DOI: 10.3390/ijms19010226
Evaluation of a New Survivin ELISA and UBC® Rapid for the Detection of Bladder Cancer in Urine
Abstract
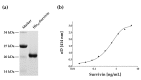
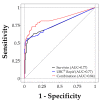
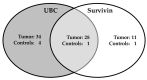
Urine-based biomarkers for non-invasive diagnosis of bladder cancer are urgently needed. No single marker with sufficient sensitivity and specificity has been described so far. Thus, a combination of markers appears to be a promising approach. The aim of this case-control study was to evaluate the performance of an in-house developed enzyme-linked immunosorbent assay (ELISA) for survivin, the UBC®Rapid test, and the combination of both assays. A total of 290 patients were recruited. Due to prior bladder cancer, 46 patients were excluded. Urine samples were available from 111 patients with bladder cancer and 133 clinical controls without urologic diseases. Antibodies generated from recombinant survivin were utilized to develop a sandwich ELISA. The ELISA and the UBC®Rapid test were applied to all urine samples. Receiver operating characteristic (ROC) analysis was used to evaluate marker performance. The survivin ELISA exhibited a sensitivity of 35% with a specificity of 98%. The UBC®Rapid test showed a sensitivity of 56% and a specificity of 96%. Combination of both assays increased the sensitivity to 66% with a specificity of 95%. For high-grade tumors, the combination showed a sensitivity of 82% and a specificity of 95%. The new survivin ELISA and the UBC®Rapid test are both able to detect bladder cancer, especially high-grade tumors. However, the performance of each individual marker is moderate and efforts to improve the survivin assay should be pursued. A combination of both assays confirmed the benefit of using marker panels. The results need further testing in a prospective study and with a high-risk population.
Keywords: UBC® Rapid; biomarker combination; bladder cancer; non-invasive; survivin; urine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Schmitz-Drager B.J., Droller M., Lokeshwar V.B., Lotan Y., Hudson M.L.A., van Rhijn B.W., Marberger M.J., Fradet Y., Hemstreet G.P., Malmstrom P.-U., et al. Molecular markers for bladder cancer screening, early diagnosis, and surveillance: The WHO/ICUD consensus. Urol. Int. 2015;94:1–24. doi: 10.1159/000369357. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

