Fast, quantitative, murine cardiac 19F MRI/MRS of PFCE-labeled progenitor stem cells and macrophages at 9.4T
- PMID: 29324754
- PMCID: PMC5764257
- DOI: 10.1371/journal.pone.0190558
Fast, quantitative, murine cardiac 19F MRI/MRS of PFCE-labeled progenitor stem cells and macrophages at 9.4T
Erratum in
-
Correction: Fast, quantitative, murine cardiac 19F MRI/MRS of PFCE-labeled progenitor stem cells and macrophages at 9.4T.PLoS One. 2019 Nov 21;14(11):e0225786. doi: 10.1371/journal.pone.0225786. eCollection 2019. PLoS One. 2019. PMID: 31751417 Free PMC article.
Abstract
Purpose: To a) achieve cardiac 19F-Magnetic Resonance Imaging (MRI) of perfluoro-crown-ether (PFCE) labeled cardiac progenitor stem cells (CPCs) and bone-derived bone marrow macrophages, b) determine label concentration and cellular load limits, and c) achieve spectroscopic and image-based quantification.
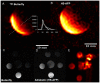
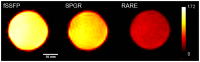
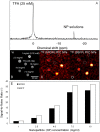
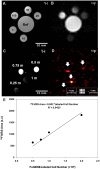
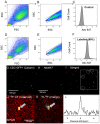
Methods: Theoretical simulations and experimental comparisons of spoiled-gradient echo (SPGR), rapid acquisition with relaxation enhancement (RARE), and steady state at free precession (SSFP) pulse sequences, and phantom validations, were conducted using 19F MRI/Magnetic Resonance Spectroscopy (MRS) at 9.4 T. Successful cell labeling was confirmed using flow cytometry and confocal microscopy. For CPC and macrophage concentration quantification, in vitro and post-mortem cardiac validations were pursued with the use of the transfection agent FuGENE. Feasibility of fast imaging is demonstrated in murine cardiac acquisitions in vivo, and in post-mortem murine skeletal and cardiac applications.
Results: SPGR/SSFP proved favorable imaging sequences yielding good signal-to-noise ratio values. Confocal microscopy confirmed heterogeneity of cellular label uptake in CPCs. 19F MRI indicated lack of additional benefits upon label concentrations above 7.5-10 mg/ml/million cells. The minimum detectable CPC load was ~500k (~10k/voxel) in two-dimensional (2D) acquisitions (3-5 min) using the butterfly coil. Additionally, absolute 19F based concentration and intensity estimates (trifluoroacetic-acid solutions, macrophages, and labeled CPCs in vitro and post-CPC injections in the post-mortem state) scaled linearly with fluorine concentrations. Fast, quantitative cardiac 19F-MRI was demonstrated with SPGR/SSFP and MRS acquisitions spanning 3-5 min, using a butterfly coil.
Conclusion: The developed methodologies achieved in vivo cardiac 19F of exogenously injected labeled CPCs for the first time, accelerating imaging to a total acquisition of a few minutes, providing evidence for their potential for possible translational work.
Conflict of interest statement
Figures


References
-
- Feyen DA, Gaetani R, Doevendans PA, Sluijter JP. Stem cell-based therapy: Improving myocardial cell delivery. Advanced Drug Delivery Reviews 2016; 106(PtA):104–115. doi: 10.1016/j.addr.2016.04.023 - DOI - PubMed
-
- Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005; 307(5709):538–544. doi: 10.1126/science.1104274 - DOI - PMC - PubMed
-
- Chen IY, Wu JC. Cardiovascular molecular imaging: focus on clinical translation. Circulation. 2011;123(4):425–43. doi: 10.1161/CIRCULATIONAHA.109.916338 - DOI - PMC - PubMed
-
- Stuckey DJ, Carr CA, Martin-Rendon E, Tyler DJ, Willmott C, Cassidy PJ, et al. Iron particles for noninvasive monitoring of bone marrow stromal cell engraftment into, and isolation of viable engrafted donor cells from, the heart. Stem Cells 2006; 24(8):1968–75. doi: 10.1634/stemcells.2006-0074 - DOI - PubMed
-
- Ahrens ET, Flores R, Xu H, Morel PA. In vivo imaging platform for tracking immunotherapeutic cells. Nature Biotechnology 2005; 23(8):983–987. doi: 10.1038/nbt1121 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

