Evidence for PMAT- and OCT-like biogenic amine transporters in a probiotic strain of Lactobacillus: Implications for interkingdom communication within the microbiota-gut-brain axis
- PMID: 29324833
- PMCID: PMC5764344
- DOI: 10.1371/journal.pone.0191037
Evidence for PMAT- and OCT-like biogenic amine transporters in a probiotic strain of Lactobacillus: Implications for interkingdom communication within the microbiota-gut-brain axis
Abstract
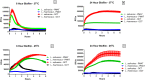
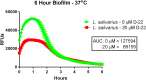
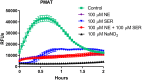
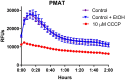
The ability of prokaryotic microbes to produce and respond to neurochemicals that are more often associated with eukaryotic systems is increasingly recognized through the concept of microbial endocrinology. Most studies have described the phenomena of neurochemical production by bacteria, but there remains an incomplete understanding of the mechanisms by which microbe- or host-derived neuroactive substances can be recognized by bacteria. Based on the evolutionary origins of eukaryotic solute carrier transporters, we hypothesized that bacteria may possess an analogous uptake function for neuroactive biogenic amines. Using specific fluorescence-based assays, Lactobacillus salivarius biofilms appear to express both plasma membrane monoamine transporter (PMAT)- and organic cation transporter (OCT)-like uptake of transporter-specific fluorophores. This phenomenon is not distributed throughout the genus Lactobacillus as L. rhamnosus biofilms did not take up these fluorophores. PMAT probe uptake into L. salivarius biofilms was attenuated by the protonophore CCCP, the cation transport inhibitor decynium-22, and the natural substrates norepinephrine, serotonin and fluoxetine. These results provide the first evidence, to our knowledge, for the existence of PMAT- and OCT-like uptake systems in a bacterium. They also suggest the existence of a hitherto unrecognized mechanism by which a probiotic bacterium may interact with host signals and may provide a means to examine microbial endocrinology-based interactions in health and disease that are part of the larger microbiota-gut-brain axis.
Conflict of interest statement
Figures
References
-
- Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303(11):G1288–95. Epub 2012/10/16. doi: 10.1152/ajpgi.00341.2012 . - DOI - PubMed
-
- Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10(8):473–86. Epub 2013/06/26. doi: 10.1038/nrgastro.2013.105 - DOI - PMC - PubMed
-
- Yang Y-X, Mu C-L, Zhang J-F, Zhu W-Y. Determination of Biogenic Amines in Digesta by High Performance Liquid Chromatography with Precolumn Dansylation. Anal Lett. 2014;47(8):1290–8. doi: 10.1080/00032719.2013.871550 - DOI
-
- Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res. 2011;343(1):23–32. Epub 2010/10/14. doi: 10.1007/s00441-010-1050-0 . - DOI - PubMed
-
- Sandrini S, Aldriwesh M, Alruways M, Freestone P. Microbial endocrinology: host-bacteria communication within the gut microbiome. J Endocrinol. 2015;225(2):R21–34. Epub 2015/03/21. doi: 10.1530/JOE-14-0615 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

