Synaptic markers of cognitive decline in neurodegenerative diseases: a proteomic approach
- PMID: 29324989
- PMCID: PMC5837272
- DOI: 10.1093/brain/awx352
Synaptic markers of cognitive decline in neurodegenerative diseases: a proteomic approach
Erratum in
-
Corrigendum.Brain. 2019 Jun 1;142(6):e24. doi: 10.1093/brain/awz049. Brain. 2019. PMID: 30838374 Free PMC article. No abstract available.
Abstract
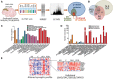
See Attems and Jellinger (doi:10.1093/brain/awx360) for a scientific commentary on this article.Cognitive changes occurring throughout the pathogenesis of neurodegenerative diseases are directly linked to synaptic loss. We used in-depth proteomics to compare 32 post-mortem human brains in the prefrontal cortex of prospectively followed patients with Alzheimer's disease, Parkinson's disease with dementia, dementia with Lewy bodies and older adults without dementia. In total, we identified 10 325 proteins, 851 of which were synaptic proteins. Levels of 25 synaptic proteins were significantly altered in the various dementia groups. Significant loss of SNAP47, GAP43, SYBU (syntabulin), LRFN2, SV2C, SYT2 (synaptotagmin 2), GRIA3 and GRIA4 were further validated on a larger cohort comprised of 92 brain samples using ELISA or western blot. Cognitive impairment before death and rate of cognitive decline significantly correlated with loss of SNAP47, SYBU, LRFN2, SV2C and GRIA3 proteins. Besides differentiating Parkinson's disease dementia, dementia with Lewy bodies, and Alzheimer's disease from controls with high sensitivity and specificity, synaptic proteins also reliably discriminated Parkinson's disease dementia from Alzheimer's disease patients. Our results suggest that these particular synaptic proteins have an important predictive and discriminative molecular fingerprint in neurodegenerative diseases and could be a potential target for early disease intervention.
Keywords: Alzheimer’s disease; Lewy body dementias; cognitive impairment; mass spectrometry; synaptic proteins.
© The Author(s) (2018). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


Comment in
-
Neurodegenerative disease: Proteome points to synaptic dysfunction in dementia.Nat Rev Neurol. 2018 Mar;14(3):128. doi: 10.1038/nrneurol.2018.5. Epub 2018 Jan 29. Nat Rev Neurol. 2018. PMID: 29377007 No abstract available.
-
Proteomics for synaptic markers of cognitive decline in neurodegenerative diseases.Brain. 2018 Feb 1;141(2):329-331. doi: 10.1093/brain/awx360. Brain. 2018. PMID: 29390123 No abstract available.
References
-
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 2003; 60: 387–92. - PubMed
-
- Aarsland D, Perry R, Brown A, Larsen JP, Ballard C. Neuropathology of dementia in Parkinson's disease: a prospective, community-based study. Ann Neurol 2005; 58: 773–6. - PubMed
-
- Abdi F, Quinn JF, Jankovic J, McIntosh M, Leverenz JB, Peskind E, et al. Detection of biomarkers with a multiplex quantitative proteomic platform in cerebrospinal fluid of patients with neurodegenerative disorders. J Alzheimers Dis 2006; 9: 293–348. - PubMed
-
- Barthelemy NR, Gabelle A, Hirtz C, Fenaille F, Sergeant N, Schraen-Maschke S, et al. Differential mass spectrometry profiles of Tau protein in the cerebrospinal fluid of patients with Alzheimer's disease, progressive supranuclear palsy, and dementia with Lewy bodies. J Alzheimers Dis 2016; 51: 1033–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

