Redox Paradox: A Novel Approach to Therapeutics-Resistant Cancer
- PMID: 29325444
- PMCID: PMC6157438
- DOI: 10.1089/ars.2017.7485
Redox Paradox: A Novel Approach to Therapeutics-Resistant Cancer
Abstract
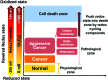
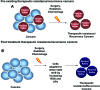
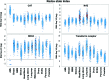
Significance: Cancer cells that are resistant to radiation and chemotherapy are a major problem limiting the success of cancer therapy. Aggressive cancer cells depend on elevated intracellular levels of reactive oxygen species (ROS) to proliferate, self-renew, and metastasize. As a result, these aggressive cancers maintain high basal levels of ROS compared with normal cells. The prominence of the redox state in cancer cells led us to consider whether increasing the redox state to the condition of oxidative stress could be used as a successful adjuvant therapy for aggressive cancers. Recent Advances: Past attempts using antioxidant compounds to inhibit ROS levels in cancers as redox-based therapy have met with very limited success. However, recent clinical trials using pro-oxidant compounds reveal noteworthy results, which could have a significant impact on the development of strategies for redox-based therapies.
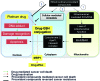
Critical issues: The major objective of this review is to discuss the role of the redox state in aggressive cancers and how to utilize the shift in redox state to improve cancer therapy. We also discuss the paradox of redox state parameters; that is, hydrogen peroxide (H2O2) as the driver molecule for cancer progression as well as a target for cancer treatment.
Future directions: Based on the biological significance of the redox state, we postulate that this system could potentially be used to create a new avenue for targeted therapy, including the potential to incorporate personalized redox therapy for cancer treatment.
Keywords: H2O2; personalized redox therapy; redox state; resistant cancer; rewired redox state.
Figures




References
-
- Abdel-Latif MM, Raouf AA, Sabra K, Kelleher D, and Reynolds JV. Vitamin C enhances chemosensitization of esophageal cancer cells in vitro. J Chemother 17: 539–549, 2005 - PubMed
-
- Ahmad IM, Aykin-Burns N, Sim JE, Walsh SA, Higashikubo R, Buettner GR, Venkataraman S, Mackey MA, Flanagan SW, Oberley LW, and Spitz DR. Mitochondrial O2*- and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J Biol Chem 280: 4254–4263, 2005 - PubMed
-
- Ahmad KA, Clement MV, Hanif IM, and Pervaiz S. Resveratrol inhibits drug-induced apoptosis in human leukemia cells by creating an intracellular milieu nonpermissive for death execution. Cancer Res 64: 1452–1459, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

