Adjuvant treatment of resectable biliary tract cancer with cisplatin plus gemcitabine: A prospective single center phase II study
- PMID: 29325521
- PMCID: PMC5765636
- DOI: 10.1186/s12885-017-3967-0
Adjuvant treatment of resectable biliary tract cancer with cisplatin plus gemcitabine: A prospective single center phase II study
Abstract
Background: Biliary tract cancer (BTC) is a dismal disease, even after curative intent surgery. We conducted this prospective, non-randomized phase II study to evaluate the feasibility and efficacy of cisplatin and gemcitabine as adjuvant treatment in patients with resected BTC.
Methods: Patients initially received gemcitabine 1000 mg/m2 alone on days 1, 8 and 15 every 28-days for a total of six cycles (single agent cohort), and after protocol amendment a combination therapy with gemcitabine 1000 mg/m2 and cisplatin 25 mg/m2 on days 1 and 8 was administered every 21 days for a total of eight cycles (combined regimen cohort). Treatment was planned to start within eight weeks after curative intent resection. Adverse events, disease-free survival and overall survival were assessed.
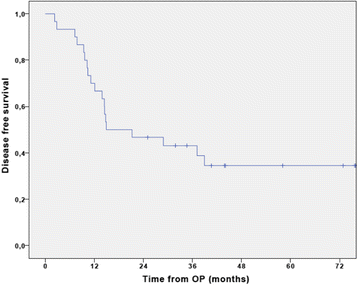
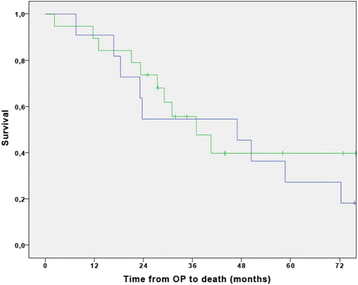
Results: Overall 30 patients were enrolled in the study from August 2008 and last patient was enrolled at 2nd December 2014. The follow-up of the patients ended at 31st December 2016. The first 9 patients received single-agent gemcitabine. The interim analysis met the predefined feasibility criteria and, from September 2010 on, the second group of 21 patients received the combination of cisplatin plus gemcitabine. In the single-agent cohort with gemcitabine the median relative dose intensity (RDI) was 100% (IQR 88.3-100). Patients treated with the combination cisplatin-gemcitabine received an overall median RDI of 100% (IQR 50-100) for cisplatin and 100% (IQR 75-100) for gemcitabine respectively. The most significant non-hematological adverse events (grade 3 or 4) were fatigue (20%), infections during neutropenia (10%), and two cases of biliary sepsis (7%). Abnormal liver function was seen in 10% of the patients. One patient died due to infectious complications during treatment with cisplatin and gemcitabine. The median disease-free survival (DFS) was 14.9 months (95% CI 0-33.8) with a corresponding 3-year DFS of 43.1 ± 9.1%. The median overall survival (OS) was 40.6 months (95% CI 18.8-62.3) with a 3-year OS of 55.7 ± 9.2%. No statistically significant differences in survival were seen between the two treatment cohorts.
Conclusion: Adjuvant chemotherapy with gemcitabine with or without cisplatin was well tolerated and resulted in promising survival of the patients.
Trial registration: The study was retrospectively registered on 25th June 2009 at clinicaltrials.gov ( NCT01073839 ).
Keywords: Adjuvant chemotherapy; Biliary tract cancer; Cholangiocellular carcinoma; Cisplatin and gemcitabine; Feasibility; Gallbladder cancer.
Conflict of interest statement
Ethics approval and consent to participate
The study was conducted after obtaining approval from the local ethical committee (ethical number KEK Zurich 1442) at 15th January 2008 and Swissmedic by 2nd April 2008. First patient was included at 4th August 2008. Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. The study was registered at clinicaltrials.gov (NCT01073839). The study was retrospectively registered on 25th June 2009 at clinicaltrials.gov, as it was not yet obligatory at the time of trial start according to national specifications.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

