Clinical research ethics review process in Lebanon: efficiency and functions of research ethics committees - results from a descriptive questionnaire-based study
- PMID: 29325585
- PMCID: PMC5765668
- DOI: 10.1186/s13063-017-2397-2
Clinical research ethics review process in Lebanon: efficiency and functions of research ethics committees - results from a descriptive questionnaire-based study
Abstract
Background: Clinical trials conducted in Lebanon are increasing. However, little is known about the performance of research ethics committees (RECs) in charge of reviewing the research protocols. This study aimed to assess the level of adherence to the ethics surrounding the conduct of clinical trials and perceptions of team members regarding roles of the RECs during the conduct of clinical trials in Lebanon. The research question was: Are RECs adherent to the ethics surrounding the conduct of clinical trials (chapters II and IV in 'Standards and Operational Guidance for Ethics Review of Health-related Research with Human Participants' in Lebanon?'
Methods: This was a quantitative and descriptive questionnaire-based study conducted among RECs of university hospitals in Lebanon. The questionnaire had to be completed online and included general questions in addition to items reflecting the different aspects of a REC performance and effectiveness. All the questionnaire was assigned a total score of 175 points. General information and questions assigned point values/scores were analysed using descriptive statistics: frequency and percentage, mean score ± standard deviation.
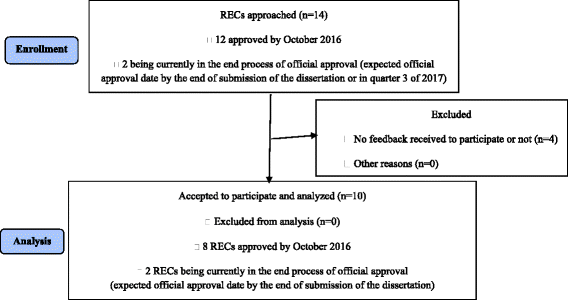
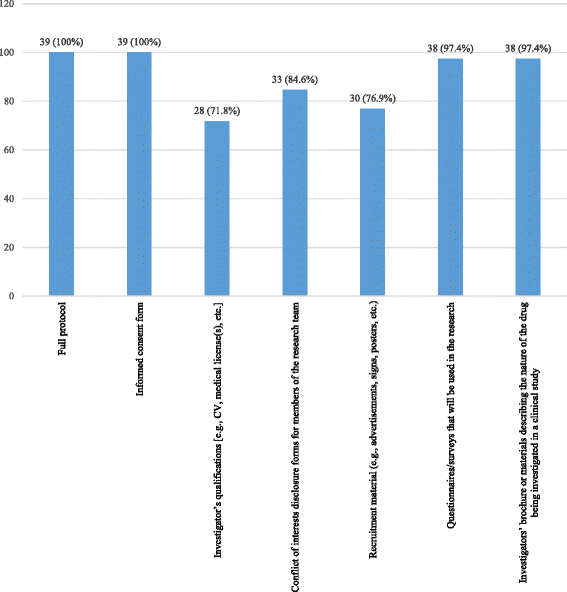
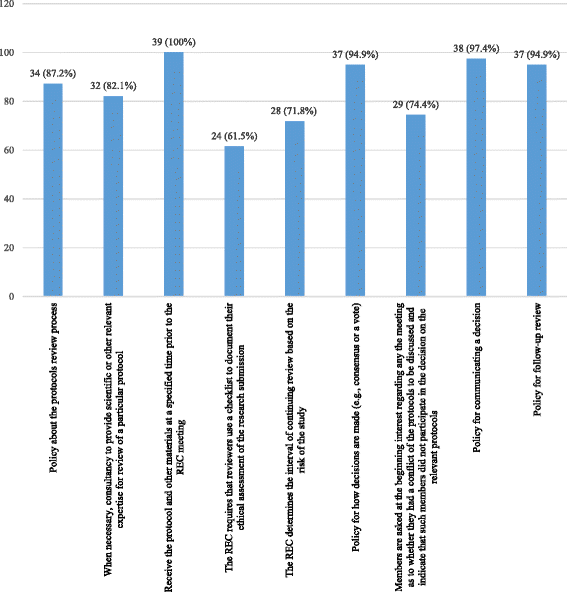
Results: Ten RECs participated in the study (52 persons: four chairs, one vice-president, 47 ordinary members). Forty-seven (90.4%) had previous experience with clinical research and 30 (57.7%) had a diploma or had done a training in research ethics. Forty-one percent confirmed that they were required to have a training in research ethics. All RECs had a policy for disclosing and managing potential conflicts of interest for its members, but 71.8% of participants reported the existence of such a policy for researchers. Thirty-three point three percent reported that the RECs had an anti-bribery policy. The questionnaire mean score was 129.6 ± 22.3/175 points reflecting thus an excellent adherence to international standards.
Conclusion: Inadequate training of REC members and the lack of anti-bribery policies should be resolved to improve their performance.
Keywords: Adherence; Effectiveness; Performance; Research ethics committees; Scores.
Conflict of interest statement
Ethics approval and consent to participate
Conducted among adult members of RECs in Lebanon, this study involves human subjects. However, no benefits or hazards are discerned in this context. Thus, no approval was required from any local ethical committee in Lebanon, except from one university medical centre in Beirut, Lebanon. In parallel, the study could have had an expedited review because there were limited risks, but the present proposal was submitted to the University of Liverpool in February 2017 for ethics clearance, which was given in March 2017. The questionnaire completion was equivalent to consenting to participate in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- MCT CRO: regional reach. 2017. http://www.mct-cro.com/regional-reach/cro-lebanon/. Accessed 23 May 2017.
-
- ClinicalTrials.gov. 2017. https://clinicaltrials.gov/ct2/results?phase=0123&cntry1=ME%3ALB. Accessed 23 May 2017.
-
- Nuffield Council on Bioethics. The ethics of research related to healthcare in developing countries. 2002. p. 189. http://nuffieldbioethics.org/wp-content/uploads/2014/07/Ethics-of-resear.... Accessed 6 Nov 2016.
-
- Council for International Organizations of Medical Sciences (CIOMS) International ethical guidelines for biomedical research involving human subjects. Geneva: CIOMS; 2002. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources