Bile Acid Metabolism in Liver Pathobiology
- PMID: 29325602
- PMCID: PMC5954621
- DOI: 10.3727/105221618X15156018385515
Bile Acid Metabolism in Liver Pathobiology
Abstract
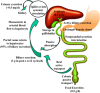
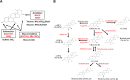
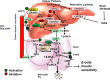
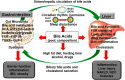
Bile acids facilitate intestinal nutrient absorption and biliary cholesterol secretion to maintain bile acid homeostasis, which is essential for protecting liver and other tissues and cells from cholesterol and bile acid toxicity. Bile acid metabolism is tightly regulated by bile acid synthesis in the liver and bile acid biotransformation in the intestine. Bile acids are endogenous ligands that activate a complex network of nuclear receptor farnesoid X receptor and membrane G protein-coupled bile acid receptor-1 to regulate hepatic lipid and glucose metabolic homeostasis and energy metabolism. The gut-to-liver axis plays a critical role in the regulation of enterohepatic circulation of bile acids, bile acid pool size, and bile acid composition. Bile acids control gut bacteria overgrowth, and gut bacteria metabolize bile acids to regulate host metabolism. Alteration of bile acid metabolism by high-fat diets, sleep disruption, alcohol, and drugs reshapes gut microbiome and causes dysbiosis, obesity, and metabolic disorders. Gender differences in bile acid metabolism, FXR signaling, and gut microbiota have been linked to higher prevalence of fatty liver disease and hepatocellular carcinoma in males. Alteration of bile acid homeostasis contributes to cholestatic liver diseases, inflammatory diseases in the digestive system, obesity, and diabetes. Bile acid-activated receptors are potential therapeutic targets for developing drugs to treat metabolic disorders.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures


References
-
- Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89(1):147–91. - PubMed
-
- Thomas C, Auwerx J, Schoonjans K. Bile acids and the membrane bile acid receptor TGR5—Connecting nutrition and metabolism. Thyroid 2008;18(2):167–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
