Induced hypothermia in patients with septic shock and respiratory failure (CASS): a randomised, controlled, open-label trial
- PMID: 29325753
- PMCID: PMC10928558
- DOI: 10.1016/S2213-2600(18)30004-3
Induced hypothermia in patients with septic shock and respiratory failure (CASS): a randomised, controlled, open-label trial
Abstract
Background: Animal models of serious infection suggest that 24 h of induced hypothermia improves circulatory and respiratory function and reduces mortality. We tested the hypothesis that a reduction of core temperature to 32-34°C attenuates organ dysfunction and reduces mortality in ventilator-dependent patients with septic shock.
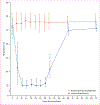
Methods: In this randomised, controlled, open-label trial, we recruited patients from ten intensive care units (ICUs) in three countries in Europe and North America. Inclusion criteria for patients with severe sepsis or septic shock were a mean arterial pressure of less than 70 mm Hg, mechanical ventilation in an ICU, age at least 50 years, predicted length of stay in the ICU at least 24 h, and recruitment into the study within 6 h of fulfilling inclusion criteria. Exclusion criteria were uncontrolled bleeding, clinically important bleeding disorder, recent open surgery, pregnancy or breastfeeding, or involuntary psychiatric admission. We randomly allocated patients 1:1 (with variable block sizes ranging from four to eight; stratified by predictors of mortality, age, Acute Physiology and Chronic Health Evaluation II score, and study site) to routine thermal management or 24 h of induced hypothermia (target 32-34°C) followed by 48 h of normothermia (36-38°C). The primary endpoint was 30 day all-cause mortality in the modified intention-to-treat population (all randomly allocated patients except those for whom consent was withdrawn or who were discovered to meet an exclusion criterion after randomisation but before receiving the trial intervention). Patients and health-care professionals giving the intervention were not masked to treatment allocation, but assessors of the primary outcome were. This trial is registered with ClinicalTrials.gov, number NCT01455116.
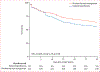
Findings: Between Nov 1, 2011, and Nov 4, 2016, we screened 5695 patients. After recruitment of 436 of the planned 560 participants, the trial was terminated for futility (220 [50%] randomly allocated to hypothermia and 216 [50%] to routine thermal management). In the hypothermia group, 96 (44·2%) of 217 died within 30 days versus 77 (35·8%) of 215 in the routine thermal management group (difference 8·4% [95% CI -0·8 to 17·6]; relative risk 1·2 [1·0-1·6]; p=0·07]).
Interpretation: Among patients with septic shock and ventilator-dependent respiratory failure, induced hypothermia does not reduce mortality. Induced hypothermia should not be used in patients with septic shock.
Funding: Trygfonden, Lundbeckfonden, and the Danish National Research Foundation.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
J-UJ reports grants from TrygFonden, Lundbeckfonden, and the Danish National Research Foundation and non-financial support from Emcools, Medivance, and Zoll during the conduct of the study and grants from Boehringer Ingelheim and non-financial support from Roche and Boehringer Ingelheim outside of the submitted work. MB reports grants from the Nordsjællands Hospital Research Fund during the conduct of the study and personal fees from Bard outside of the submitted work. All other authors declare no competing interests.
Figures

Comment in
-
Body temperature in sepsis: a hot topic.Lancet Respir Med. 2018 Mar;6(3):162-163. doi: 10.1016/S2213-2600(18)30003-1. Epub 2018 Jan 8. Lancet Respir Med. 2018. PMID: 29325752 No abstract available.
References
-
- Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ 2016; 353: i1585. - PubMed
-
- Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014; 312: 90–92. - PubMed
-
- McCloskey RV, Straube RC, Sanders C, Smith SM, Smith CR. Treatment of septic shock with human monoclonal antibody HA-1A. A randomized, double-blind, placebo-controlled trial. CHESS Trial Study Group. Ann Intern Med 1994; 121: 1–5. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

