Explanations for the high potency of HPV prophylactic vaccines
- PMID: 29325819
- PMCID: PMC6035892
- DOI: 10.1016/j.vaccine.2017.12.079
Explanations for the high potency of HPV prophylactic vaccines
Abstract
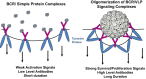
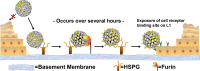
HPV L1 virus-like particle (VLP) vaccines administered in a prime/boost series of three injections over six months have demonstrated remarkable prophylactic efficacy in clinical trials and effectiveness in national immunization programs with high rates of coverage. There is mounting evidence that the vaccines have similar efficacy and effectiveness even when administered in a single dose. The unexpected potency of one dose of these VLP vaccines may largely be attributed to structural features of the particles, which lead to the efficient generation of long-lived antigen-specific antibody-producing cells and unique features of the virus life cycle that make the HPV virions highly susceptible to antibody-mediated inhibition of infection.
Keywords: HPV; Human papillomavirus; Prophylactic vaccine; Virus-like particle.
Copyright © 2018. Published by Elsevier Ltd.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

