Treatment Effect of Drug-Coated Balloons Is Durable to 3 Years in the Femoropopliteal Arteries: Long-Term Results of the IN.PACT SFA Randomized Trial
- PMID: 29326153
- PMCID: PMC5771683
- DOI: 10.1161/CIRCINTERVENTIONS.117.005891
Treatment Effect of Drug-Coated Balloons Is Durable to 3 Years in the Femoropopliteal Arteries: Long-Term Results of the IN.PACT SFA Randomized Trial
Abstract
Background: Randomized controlled trials have reported favorable 1-year outcomes with drug-coated balloons (DCBs) for the treatment of symptomatic peripheral arterial disease when compared with standard percutaneous transluminal angioplasty (PTA). Evidence remains limited on the durability of the treatment effect with DCBs in the longer term.
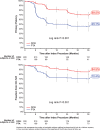
Methods and results: IN.PACT SFA is a single-blind, randomized trial (Randomized Trial of IN.PACT Admiral Paclitaxel-Coated Percutaneous Transluminal Angioplasty [PTA] Balloon Catheter vs Standard PTA for the Treatment of Atherosclerotic Lesions in the Superficial Femoral Artery [SFA] and/or Proximal Popliteal Artery [PPA]) that enrolled 331 patients with symptomatic (Rutherford 2-4) femoropopliteal lesions up to 18 cm in length. Patients were randomized 2:1 to receive treatment with DCB or PTA. The 36-month assessments included primary patency, freedom from clinically driven target lesion revascularization, major adverse events, and functional outcomes. At 36 months, primary patency remained significantly higher among patients treated with DCB compared with PTA (69.5% versus 45.1%; log rank P<0.001). The rates of clinically driven target lesion revascularization were 15.2% and 31.1% (P=0.002) for the DCB and PTA groups, respectively. Functional outcomes were similarly improved between treatment groups even though subjects in the DCB group required significantly fewer reinterventions versus those in the PTA group (P<0.001 for target lesion revascularization, P=0.001 for target vessel revascularization). There were no device- or procedure-related deaths as adjudicated by an independent Clinical Events Committee.
Conclusions: Three-year results demonstrate a durable and superior treatment effect among patients treated with DCB versus standard PTA, with significantly higher primary patency and lower clinically driven target lesion revascularization, resulting in similar functional improvements with reduced need for repeat interventions.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifiers: NCT01175850 for IN.PACT SFA phase I in the European Union and NCT01566461 for IN.PACT SFA phase II in the United States.
Keywords: angioplasty; peripheral arterial disease; target lesion revascularization.
© 2018 The Authors.
Figures
Comment in
-
Leave No Stent Behind: Are Drug-Coated Balloons Enough for Femoropopliteal Disease?Circ Cardiovasc Interv. 2018 Jan;11(1):e006285. doi: 10.1161/CIRCINTERVENTIONS.117.006285. Circ Cardiovasc Interv. 2018. PMID: 29326156 No abstract available.
-
Letter by Dan et al Regarding Article, "Treatment Effect of Drug-Coated Balloons Is Durable to 3 Years in the Femoropopliteal Arteries: Long-Term Results of the IN.PACT SFA Randomized Trial".Circ Cardiovasc Interv. 2018 Jun;11(6):e006679. doi: 10.1161/CIRCINTERVENTIONS.118.006679. Circ Cardiovasc Interv. 2018. PMID: 29895602 No abstract available.
-
Response by Schneider et al to Letter Regarding Article, "Treatment Effect of Drug-Coated Balloons Is Durable to 3 Years in the Femoropopliteal Arteries: Long-Term Results of the IN.PACT SFA Randomized Trial".Circ Cardiovasc Interv. 2018 Jun;11(6):e006699. doi: 10.1161/CIRCINTERVENTIONS.118.006699. Circ Cardiovasc Interv. 2018. PMID: 29895603 No abstract available.
References
-
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. - PubMed
-
- Dick P, Wallner H, Sabeti S, Loewe C, Mlekusch W, Lammer J, Koppensteiner R, Minar E, Schillinger M. Balloon angioplasty versus stenting with nitinol stents in intermediate length superficial femoral artery lesions. Catheter Cardiovasc Interv. 2009;74:1090–1095. doi: 10.1002/ccd.22128. - PubMed
-
- Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, Schlager O, Cejna M, Lammer J, Minar E. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–1888. doi: 10.1056/NEJMoa051303. - PubMed
-
- Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, Dave R, Ansel G, Lansky A, Cristea E, Collins TJ, Goldstein J, Jaff MR RESILIENT Investigators. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv. 2010;3:267–276. doi: 10.1161/CIRCINTERVENTIONS.109.903468. - PubMed
-
- Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, Dave R, Ansel G, Lansky A, Cristea E, Collins TJ, Goldstein J, Cao AY, Jaff MR RESILIENT Investigators. Nitinol stent implantation vs. balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: three-year follow-up from the RESILIENT randomized trial. J Endovasc Ther. 2012;19:1–9. doi: 10.1583/11-3627.1. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

